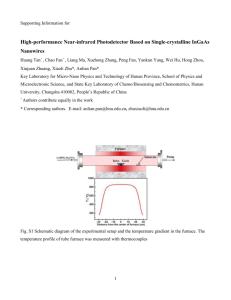
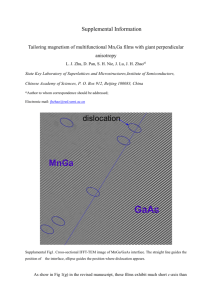
Template for Electronic Submission to ACS Journals
advertisement
Supporting Information Doping and Electronic Properties of GaAs Grown by Close-Spaced Vapor Transport from Powder Sources for Scalable III-V Photovoltaics Andrew J. Ritenour,a Jason W. Boucher, b Robert DeLancey, b Ann L. Greenaway, a Shaul Aloni, c and Shannon W. Boettcher a a Department of Chemistry and Biochemistry, and bDepartment of Physics, University of Oregon, Eugene, 97403, USA; cThe Molecular Foundry, Lawrence Berkeley National Laboratory, Berkeley, California 94720, USA e-mail: swb@uoregon.edu 1. Measuring the dopant concentrations in GaAs films 1.1 Quantification of sulfur impurity by TOF-SIMS M/Z data was collected vs scan number as the sample was sputtered with a Cs+ ion source. This data was converted to M/Z vs depth after measuring the final sputter crater depth via optical profilometry. We assume a constant sputter rate which is reasonable considering the total count-rate was steady. The concentration of S in the film and substrate was determined by integrating the total counts of the 32S peak for the film and substrate (Fig. S1), integrating the total counts of the 75As peak for the film and substrate, and converting counts into concentration using the RSF equation (e) in the main text.1 In our previous reports films were grown from graphite heaters which were purified by repeated temperature cycles in our CSVT reactor (i.e. growing GaAs films).2 Those films were subsequently determined to be S-doped by SIMS analysis (Fig. S1). GaAs films used in this study also contain S in reduced concentration (see section 1.2). Figure S1. (A) 32S region and (B) 28Si region of the mass spectrum for a CSVT film deposited on a Si-doped n+-GaAs substrate. The mass spectra indicate that the CSVT films are S-doped, not doped by diffusion of Si atoms from the substrate. 1.2 Identification of the source of the S impurity Mass spectra of the graphite were measured for as-received “high-purity graphite” (Graphite Machining, Inc.), a piece of one of the heaters after ~100 growth cycles, and high-purity graphite which was subjected to additional purification (externally by Advanced Carbon Technologies). Although we do not know an RSF for S in graphite and cannot calculate an absolute concentration, qualitatively the sulphur appears in the greatest concentration for the “as-received” sample, reduced concentration for the “after ~100 thermal cycles” sample, and has the least concentration after the additional purification (Fig. S2). We hypothesize that the S present in the graphite heaters is volatilized during heating and contributes to the S impurity which is observed in the CSVT films. We observe that our CSVT films produced before purification of the graphite heaters possessed N D = [S] = 1-2×1017 cm-3 (Fig. S1).2 After purification the CSVT films possessed 3-7×1016 cm-3. This is consistent with our hypothesis that the S impurity originates in the graphite heaters. Figure S2. Mass spectrum of the 32S region for the graphite heaters as received, after ~100 growth cycles, and after additional purification. 1 1.3 Identification and Quantification of Te doping by SIMS Identification of Te was accomplished by comparing the isotope distribution observed in the mass spectrum to the natural isotope distribution (Fig. S3). This is an important check because at high M/Z ranges there is increased possibility of misidentification due to charged fragments consisting of clusters of atoms with the same M/Z. The concentration of Te was determined Figure S3. (A) Te distribution observed in SIMS. (B) natural isotopic distribution of Te. using the same RSF calculation technique described above (Section 1.1), using the 130Te isotope peak. Depth profiles such as shown in Fig. 2 in the text were reconstructed from the mass spectra by integrating multiple sequential scans and converting counts to concentration using the RSF technique, and the scan number to depth based optical profilometry measurements. 1.4 Mott-Schottky Measurements n-Type GaAs electrodes were placed in the ferrocene/ferrocenium solution (see Methods) in the dark. The DC voltage of the GaAs sample was stepped from 0 ~ 1 V vs Esol in 10 steps. At each step the imaginary and real impedance were measured by applying a 10 mV AC bias superimposed on the DC bias. The AC frequency was swept from 500 kHz to 100 Hz in 10 steps. Data was selected from the region where the phase angle was less than -80 °, indicating the measured impedance was primarily due to the GaAs depletion region capacitance (Fig. S4B). We fit the impedance vs voltage response to an equivalent circuit (Fig. S4A) using the Bio-Logic zFit utility in order to extract the capacitance-voltage response. Then we extracted the free carrier density (ND or NA) and built-in voltage (Vbi) from Mott-Schottky plots (Fig. S5). The slope is proportional to the dopant density and the x-intercept is equal to Vbi. For GaAs in ferrocene/ferrocenium we extract Vbi ~1 V, consistent with the results of others.3 Measurements were conducted in a similar manner for p-type samples except for the following differences: p-GaAs was measured using the non-aqueous I-/I3- solution in acetonitrile (see Methods), the DC voltage was stepped from E = 0 ~ -0.5 V vs Esol in 10 steps, and the Vbi was ~0.3 V.4 Figure S4. (A) Equivalent circuit used to extract junction capacitance from electrochemical impedance measurements. (B) Example of typical Z and phase angle response to DC voltage. Each colored curve represents a different DC bias. Figure S5. Representative Mott-Schottky plots for (A) p-GaAs and (B) n-GaAs. 2 2. Spectral response of n-GaAs and p-GaAs Measurement of spectral response of n-GaAs was carried out using the standard non-aqueous ferrocene/ferrocenium electrolyte, which is known to yield reliable quantum efficiency data.3 Measurement of spectral response of p-GaAs was done using I-/I3- electrolyte in acetonitrile rather than the standard aqueous solution. Although the p-GaAs was stable in aqueous solution while illuminated,4 we observed etching of the surface (indicated by specular surfaces becoming “matte”) when the electrodes where left in solution in the dark for multiple hours. This was problematic for spectral response measurements, which were measured using a chopped light source. Measuring p-GaAs samples with the standard aqueous solution resulted in non-physical internal quantum efficiency Φint > 1. We suspect the nA-range anodic dark current caused by dissolution of the p-GaAs in the dark, having the opposite sign of the cathodic photocurrent, contributed to the amplitude of the signal measured by the lock-in amplifier, resulting in Φint > 1. Furthermore, “matte” electrode surfaces are known to enhance the external quantum efficiency Φext of photoelectrodes by reducing reflective losses and could have contributed to the inconsistent data.5 We confirmed that the signal measured in spectral response measurements using non-aqueous I-/I3- electrolyte was entirely due to photocurrent rather than galvanic current by measurements using a monochromatic 850 nm LED. The J-E response of photoanodes measured with a potentiostat was free of transients, scaled linearly with light intensity under 103-fold higher light intensity, and was equivalent to the Φint obtained from nA-range spectral response measurements using a lock-in amplifier (Fig. S6A). We also measured the photocurrent of the p-GaAs/nonaqueous I-/I3- system using the solar simulator and a potentiostat (Fig. S6B). The equivalent series resistance is significantly higher than with the aqueous electrolyte under mW-range excitation (as indicated by the low fill-factor), but is sufficient for nW-range spectral response measurements and scales linearly with light intensity. Figure S6. (A) Quantum efficiency vs applied bias of a p-GaAs sample measured in nonaqueous I-/I3- under 850 nm illumination provided by a chopped LED. The signal is free of transients and agrees with spectral response measurements done using a lock-in amplifier. (B) J-E response of a p-GaAs sample in non-aqueous I-/I3- measured under a solar simulator. The photocurrent scales with light intensity and is ~0 mA in the dark. 3. EBIC measurement of GaAs In a hypothetical “ideal” EBIC experiment the excitation volume is small with respect to LD, and the measured signal is dominated by bulk recombination rather than surface recombination. This is not the case with unpassivated GaAs. We found that adjusting the accelerating voltage used for EBIC measurement strongly influenced the sample response and resulting values of LD (Fig. 5 in the text). This is likely related to the high surface recombination velocity of unpassivated GaAs. For low accelerating voltages (< 10 keV) carriers are primarily excited near the GaAs surface where they quickly recombine and result in an err oneously low value of LD. The measured LD at high accelerating voltages (> 15 keV) is erroneously high because the excitation volume is large with respect to the LD. We passivated GaAs surfaces for EBIC measurement by applying a 1 M aqueous solution of Na2S for 30 s, rinsing with H2O, rinsing with isopropyl alcohol, and drying with N 2. After passivation, there was little dependence of LD on the accelerating voltage used for EBIC even at low voltages. The log(I) vs distance curves were linear over several orders of magnitude in I (Fig. S8) and fit to obtain the LD reported in Fig. 6B in the text. 3 Figure S7. EBIC decays with linear fits used to extract LD. These were obtained from passivated GaAs samples measured with low accelerating voltages of 2-5 keV. References (1) Wilson, R. G.; Stevie, F. A.; Magee, C. W. Secondary ion mass spectrometry: a practical handbook for depth profiling and bulk impurity analysis; Wiley New York, 1989. (2) Ritenour, A. J.; Cramer, R. C.; Levinrad, S.; Boettcher, S. W. ACS Appl. Mater. Interfaces 2011, 4, 69. (3) Gronet, C. M.; Lewis, N. S. Appl. Phys. Lett. 1983, 43, 115. (4) Fan, F. R. F.; Bard, A. J. J. Am. Chem. Soc. 1980, 102, 3677. (5) Parkinson, B. A.; Heller, A.; Miller, B. J. Electrochem. Soc. 1979, 126, 954. 4