homeworks/ChE 204 HW-4 and HW-5 together , Spring 2014, see
advertisement

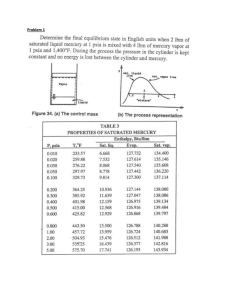
METU, Chemical Engineering Department ChE 204 Thermodynamics I (Section 06) Prof. Dr. Tülay Özbelge Asst. Emine Kayahan 20/03/2014 HOMEWORK (4 & 5) (Due Dates: March 28, 2014 for HW-4 and April 1, 2014 for HW-5) Study to solve all these problems, but submit only Problems 2, 4, 5, 8, 10, 14 on due date of March 28, 2014. Submit the solutions of Problems 16, 17, 18 on due date of April 1, 2014. Problem 1. A 10 m3 tank contains nitrogen at 300C and 900 kPa. Some nitrogen is allowed to escape until the pressure in the tank drops to 650 kPa. If the temperature at this point is 250C, determine the amount of nitrogen that has escaped. Assume that nitrogen is an ideal gas. Problem 2. Two moles of N2 (ideal gas, molecular weight: 28 g / gmol) at 10 bar and 330 K (state 1) undergoes the following reversible changes: (a) Compressed isothermally to 14 bar (state 2). (b) Heated at constant pressure to 660 K (state 3). (c) Cooled at constant volume until the pressure drops to 6 bar (state 4), (d) Compresses adiabatically to 10 bar (state 5), (e) Heated at constant pressure to state 1 (i) Sketch the paths followed in each process on a single P-V diagram, (ii) Calculate the work required, the heat transferred, and the changes in internal energy and enthalpy for each of the five processes and for the entire cycle. Note: You may assume constant heat capacity of Cp = 29.3 J / (mol.K) Problem 3. A torque in newton-meters required rotate a shaft through an angle of θ is given by the equation T = 500 (1+sin θ) What size motor, in kilowatts, is required to turn the crank at an average speed of 1000 rev/min? Determine the work required to rotate the crank through one revolution. Problem 4. An insulated and non-conducting container filled with 10 kg of water at 200C is fitted with a stirrer. The stirrer is made to turn by gravity acting on a weight mass of 2.5 kg. The weight falls slowly through a distance of 10 m in driving the stirrer. Assuming that all work done on the weight is transferred to the water and that the local acceleration of gravity is 9.8 m/s2. Determine: (a) The amount of work done on the water, (b) The internal-energy change of the water, (c) The final temperature of the water, (d) The amount of heat that must be removed from the water to return it to its initial temperature, (e) The total energy change of the universe because of (1) the process of lowering the weight, (2) the process of cooling the water back to its initial temperature, and (3) both process together. Problem 5. An insulated piston cylinder device contains 5 L of saturated liquid water at a constant pressure of 150 kPa. Water is stirred by a paddle wheel while a current of 8 A flows for 45 min through a resistor placed in the water. If one-half of the liquid is evaporated during this constant pressure process and the paddle-wheel work amounts to 300 kJ, determine the voltage of the source. Also, show the process on a P-V diagram with respect to saturation lines. Problem 6. Nitrogen flows at steady state through a horizontal, insulated pipe with inside diameter of 5.08 cm. A pressure drop results from flow through a partially opened valve. Just upstream from the valve the pressure is 551.6 kPa, the temperature is 37.80C, and the average velocity is 4.57 m/s. If the pressure just downstream from the valve is 137.9 kPa, what is the temperature? Assume for nitrogen that PV/T=constant, CV=(5/2) R and CP=(7/2) R. Problem 7. An insulated piston-cylinder device initially contains 0.3 m3 of carbon dioxide at 200 kPa and 27C. An electric switch is turned on, and a 110-V source supplies current to a resistance heater inside the cylinder for a period of 10 min. The pressure is held constant during the process, while the volume is doubled. Determine the current that passes through the resistance heater. Carbon dioxide can be considered to be an ideal gas with Cp* = 37 J/mol K. Problem 8. Air at 80 kPa and 127C enters an adiabatic diffuser steadily at a rate of 6000 kg/h and leaves at 100 kPa. The velocity of the air stream is decreased from 230 to 30 m/s as it passes through the diffuser. Find: (a) the exit temperature of the air, (b) the exit area of the diffuser. Air is assumed to be an ideal gas with Cp* = 30 J/mol K. Problem 9. One mole of an ideal gas, initially at 50C and 2 bar, is changed to 140C and 9 bar by three different mechanically reversible processes: The gas is first heated at constant volume until its temperature is 140C; then it is compressed isothermally until its pressure is 9 bar. The gas is first heated at constant pressure until its temperature is 140C; then it is compressed isothermally until its pressure is 9 bar. The gas is first compressed isothermally to 9 bar; then it is heated at constant pressure to 140C. Show the process diagram as P versus V, for each of the above cases. Calculate Q, W, U, and H in each case. Take Cp* = (7/2)R and Cv = (5/2)R. Problem 10. The mixing tank shown in the figure initially contains 50 kg of water at 25C. Suddenly the two inlet valves and the single outlet valve are opened, so that two water streams, each with a flow rate of 5 kg/min, flow into the tank, and a single exit stream with a flow rate of 10 kg/min leaves the tank. The temperature of one inlet stream is 80C, and that of the other is 50C. The tank is well mixed, so that the temperature of the outlet stream is always the same as the temperature of the water in the tank. Fig.1 (a) Compute the steady-state temperature that will finally be obtained in the tank. (b) Develop an expression for the temperature of the fluid in the tank at any time. Problem 11. Nitrogen gas is being withdrawn at the rate of 4.5 g/s from a 0.15-m3 cylinder, initially containing the gas at a pressure of 10 bar and 320 K. The cylinder does not conduct heat, nor does its temperature change during the emptying process. What will be the temperature and pressure of the gas in the cylinder after 5 minutes? What will be the rate of change of the gas temperature at this time? Nitrogen can be considered to be an ideal gas with Cp*= 30 J/(mol K). Problem 12. A 0.01-m3 cylinder containing nitrogen gas initially at a pressure of 200 bar and 250 K is connected to another cylinder 0.005 m3 in volume, which is initially evacuated. A valve between the two cylinders is opened until the pressures in the cylinders equalize. Find the final temperature and pressure in each cylinder if there is no heat flow into or out of the cylinder. You may assume that there is no heat transfer between the gas and the cylinder walls and that the gas is ideal with a constant-pressure heat capacity of 30 J/(mol K). Problem 13. Repeat the calculation of Problem 12, but now assume that sufficient heat transfer occurs between the gas in the two cylinders that both final temperatures and both final pressures are the same. Problem 14. A rigid tank is divided into two chambers A and B by a membrane. The volume of chamber A is 1 m3. Initially chamber A contains steam at 175 kPa with a specific volume of 1.0 m3/kg and chamber B contains 3.5 kg steam at 0.5 MPa and 400 C. The membrane now ruptures and heat transfer takes place so the whole system comes to a uniform state at 100C. Find the heat transferred during the process. Problem 15. An insulated tank is divided into two parts by a partition as shown in Fig. 1. One part of the tank contains 2.5 kg of compressed liquid water at 60C and 600 kPa while the other part is evacuated. The partition is now removed and the water expands to fill the entire tank. Determine the final temperature of the water and the volume of the tank for a final pressure of 10 kPa. Evacuate dpartition H2O Fig. 2 Problem 16. One kilogram of steam contained in a horizontal frictionless piston and cylinder is heated at constant pressure of 1.013 bar from 125C to such a temperature that its volume doubles. Calculate: (a) the amount of heat that must be added to accomplish this change, (b) the final temperature of the steam, (c) the work done by steam on surroundings, (d) the internal energy and enthalpy changes of steam for this process. Problem 17. A non-conducting tank of negligible heat capacity and 1 m3 volume is connected to a pipeline containing steam at 5 bar and 370C, filled with steam to a pressure of 5 bar, and disconnected from the pipeline. (a) If the tank is initially evacuated, how much steam is in the tank at the end of the filling process, and what is its temperature? (b) If the tank initially contains steam at 1 bar and 150C, how much steam is in the tank at the end of the filling process, and what is its temperature? Problem18. An isolated chamber with rigid walls is divided into two equal compartments, one containing gas and the other evacuated. The partition between the compartments ruptures. After the passage of a sufficiently long period of time, the temperature and pressure are found to be uniform throughout the chamber. (a) If the filled compartment initially contains an ideal gas of constant heat capacity at 1 MPa and 500 K, what are the final temperature and pressure in the chamber? (b) If the filled compartment initially contains steam at 1 MPa and 500 K, what are the final temperature and pressure in the compartment? Problem 19. A tank contains 20% liquid water and 80% steam by volume at 200C. Steam is withdrawn from the top of the tank until the fluid remaining in the tank is at a temperature of 150C. Assuming the tank is adiabatic and only vapor is withdrawn compute: (a) The final pressure in the tank. (b) The fractions of vapor and liquid in the tank finally. (c) The fraction of the total water present initially that was withdrawn. Problem 20. A pressure cooker is a pan that cooks food much faster than ordinary pans by maintaining a higher pressure and temperature during cooking. The pressure inside the pan is controlled by a pressure regulator (the petcock) that keeps the pressure at a constant level by periodically allowing some steam to escape, thus preventing any excess pressure build-up. A certain pressure cooker has a volume of 6 L and an operating pressure of 75 kPa gage. Initially, it contains 1 kg of water. Heat is supplied to the pressure cooker at a rate of 500 W (Watt) for 30 min after the operating pressure is reached. Assuming an atmospheric pressure of 100 kPa, determine: (a) The temperature at which cooking takes place, (b) The amount of water left in the pressure cooker at the end of the process.