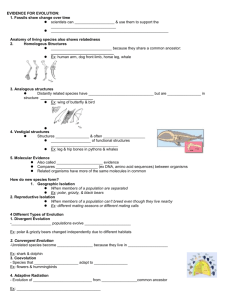
transcription observed
advertisement

Strong association of interleukin-6 -174 G>C promoter polymorphism with increased risk for oral cancer Eleftherios Vairaktaris1, Athanasios Yiannopoulos2, Antonis Vylliotis1, Christos Yapijakis1, Spyridoula Derka1, Stavros Vassiliou1, Emeka Nkenke3, Zoi Serefoglou1, Vasilis Ragos1, Giorgos Papageorgiou1, Eleni Vorris1, Elena Critseli4, Dimitris Avgoustidis1, Friedrich Neukam3, Efstratios Patsouris5 1. Department of Maxillofacial Surgery, University of Athens Medical School, Vas. Sofias 93 & Dim. Soutsou 1, Athens 11521, Greece 2. Department of A’ Surgical Clinic, University of Athens Medical School, Mikras Asias 75, Athens 11527, Greece. 3. Department of Oral and Maxillofacial Surgery, Universität Erlangen, Klinik und Poliklinik fűr Mund-, Kiefer-, Gesischtschirurgie, Glueckstrasse 11, Erlangen D91054, Nűrnberg, Germany. 4. 5. Department of Pathology, University of Athens Medical School, Mikras Asias 75, Athens 11527, Greece. Short running title: Association of interleukin-6 with oral cancer Correspondence to: Dr. Eleftherios Vairaktaris, MD,DDS,PhD,PhD Department of Oral and Maxillofacial Surgery, University of Athens Medical School, Vas. Sofias 93 & Dim. Soutsou 1, Athens 11521, Greece. Tel: +30-210-6443035; Fax: +30-210-6443803; Email: lvairakt@med.uoa.gr Source of support: This work was co-funded by the European Social Fund and National Resources (EPEAEK II “Pythagoras” 70/3/7391) grant to E.V. 1 Abstract In light to recently found contribution of factors associated with thrombosis and inflammation to carcinogenesis, we investigated the possible association of interleukin-6 (IL-6) with increased risk for oral cancer. In DNA samples of 162 patients with oral squamous cell carcinoma and 156 healthy controls of comparable ethnicity, age and sex, we studied the -174 G>C polymorphism in the IL-6 gene, which affects its transcription. The C allele frequencies were significantly increased in patients compared to controls, 42.6% versus 23.1% (P<0.001), respectively. The CC homozygotes had a seven-fold greater risk for developing oral cancer (odds ratio 7.39, 95% C.I. 2.61-20.92), while the GC heterozygotes had a four-fold greater risk (odds ratio 3.74, 95% C.I. 2.29-6.11). A significant increase of C alleles was observed in patients regardless their smoking or alcohol consumption habits, early or advanced stage of cancer, presence or absence of a family history for cancer or thrombophilia (Fischer values P<0.001). These findings suggest that the -174 G>C polymorphism, by affecting the IL-6 gene expression, is associated with aggressive progress of oral oncogenesis. 2 Introduction Oral squamous cell carcinoma (OSCC) is one of the most common human malignancies, characterised by low survival rate, which makes this disease a serious public health problem (1). Oral carcinogenesis is a multistep process occurring due to environmental factors such as tobacco and alcohol abuse and gene alterations in oncogenes and tumor suppressor genes (2). Recently, common polymorphisms in angiogenesis, inflammation and thrombosis-related genes have been associated with increased risk for oral cancer (3-7). One such factor related with both thrombosis and malignancies is interleukin 6 (IL-6) (8-11). IL-6, a phosphorylated glycoprotein containing 185 amino acids, is a pleiotropic cytokine involved in many physiological processes, including inflammation, bone metabolism, synthesis of C-reactive protein (CRP) and hematopoiesis (12). IL-6 and related cytokines have also been found to induce cachexia in cancer patients by altering metabolism of lipids and proteins. Furthermore, IL-6 functions as a growth-regulating factor in several human tumors, including plasmacytoma, melanoma, and gastric, ovarian and renal cell carcinoma (12). This particular role of IL-6 in neoplastic disease is supported by the observation that circulating levels of the cytokine increase markedly during development and progression of tumors, including those of the breast, pancreas, gastrointestinal tract, lung, ovary, prostate, and others (9-11). A single nucleotide polymorphism, upstream of the transcription start site of the IL-6 gene, involving substitution of cytosine (C) for guanine (G) (henceforth referred to as IL-6 -174G>C) affects gene expression and is associated with differences in plasma IL-6 levels (13). Other polymorphisms at adjacent sites -597, -572 and -373 also seem to 3 affect the IL-6 promoter activity, but they are in linkage disequilibrium with the polymorphism at position -174 (13). The frequency of the less common C allele ranges between 23-42 %, in various populations (14,16). The majority of studies indicate that IL-6 gene expression is constitutively greater when the more common G allele is present (14,16). Nevertheless, the IL-6 gene expression increases after an inflammatory stimulus when the C allele is present, such as in individuals with chronic inflammations or older age (17). It has been suggested that the important determinant of plasma IL-6 concentration is simply not the peak value after an inflammatory stimulus, but rather the time taken for activity to return to basal levels after stimulation (13). In subjects with chronic inflammatory activity such as smokers, patients with cardiovascular disease or elderly populations, the association between IL-6 genotype and circulating IL-6 may be the converse of that observed in young healthy populations or middle-aged populations without inflammation (13). Consistent with this, the IL-6 production of peripheral blood mononuclear cells from C allele carriers increased with age whereas this phenomenon was not observed in the GG genotype (13). In sum, while it seems clear that the -174G>C polymorphism has clearly an impact on IL-6 gene expression, the exact mechanisms remain to be delineated. Interestingly, several studies report elevated IL-6 in serum, saliva and tumor biopsies obtained from patients with oral cancer (18-19). In light of the above, we studied the IL-6 -174 G>C polymorphism in patients with oral cancer and healthy controls in order to determine whether this polymorphism is associated with increased risk for this type of cancer. 4 Materials and Methods The individuals under study were 318 unrelated Greeks and Germans, consisting of 162 patients with oral squamous cell carcinoma and 156 healthy blood donors of similar age, ethnicity and sex. The patients were mostly men (N=130) and their age ranged between 40-84 years (58.5±10.1 years, median 58.5 years). The sex ratio of the controls (N=120 men) and their age (ranged 31-83 years; 55.5±11.9 years, median 55.5 years) were comparable to those of the patients. The patients, who were included in this study, had developed oral cancer and were operated recently or up to a decade ago. In addition to clinical presentation, a biopsy with pathological diagnosis of tumor stages I-IV and a family history regarding cancer and thrombophilia were available. Sixty of them (37%) had one or two first degree relatives with cancer and their age range (median=58.7 years) did not differ significantly from the whole group of patients. Furthermore, thirty two patients (19.8 %) had one or two firstdegree relatives with idiopathic thrombosis and an earlier age range (median =58 years) but again with no statistical difference compared to the whole group. Sixteen patients (9.9%) had a positive family history for both cancer and thrombophilia (median age=56.3 years). Most of the participants in the two groups generally, worked in a low-risk environment (with the exception of one patient and three controls who worked in chemical factories). No data were available on controls regarding their family history or smoking and alcohol consumption habits. Blood samples were collected from patients and controls under study after informed consent. DNA was isolated from blood with the use of Nucleon TM kit 5 (Amersham). Molecular detection of the (-174G/C) polymorphism in the IL-6 gene was performed by restriction fragment length polymorphism typing. This involved a combination of PCR amplification and digestion with restriction endonuclease Nla III followed by gel electrophoretic analysis. The PCR conditions consisted of an initial denaturation step at 95 oC, followed by 35 cycles of 94 oC for 55 sec, 61 oC for 1 min, and 72 oC for 50 sec, as well as a final elongation step at 72 oC for 5 min. The primers used were Forward: 5’-TGACGACCTAAGCTGCACTTTTC-3’ and Reverse: 5’- GGGCTGATTGGAAACCTTATTAAGA-3’. The generated PCR product of 93 bp was cleaved by restriction enzyme Nla III into two fragments of 52bp and 41bp only if the C allele was present (Fig 1). The frequencies of alleles and genotypes of the whole group or subgroups of patients were compared to the respective frequencies of the control group using the chi square or Fisher’s exact test and odds ratios. The significance level was set at P<0.05 and the results are presented as hazard ratios with 95% confidence intervals (CI). For the purpose of statistical analysis all unknown variables of controls were assumed to be nil, thus, odds ratios obtained for some subgroups of patients may overestimate the true likehood of IL-6 genotypes and those variables. Results The prevalence of detected IL-6 genotypes in healthy controls and patients with oral cancer are shown in Tables 1-3. The data for the two tested populations (Greek and German healthy controls) were analyzed together, since there were no significant 6 differences of allele frequencies of the (-174G/C) polymorphism among the two populations. For -174G>C IL-6 promoter polymorphism, genotype distributions and allele frequencies in the control group, in patients group and subgroups were as expected for a sample in Hardy-Weinberg equilibrium. Compared to the control group, the heterozygotes and the homozygotes for the C allele were significantly increased in the group of patients and in every subgroup (P<0.001). The observed C allele frequency in the control group was 23.1%, therefore similar to other European populations. The detected C allele frequencies, in the patients’ group and in every subgroup were significantly elevated in comparison to the control group (value P<0.001), with the only exception being the patients with alcohol abuse where no significant difference was observed (Table 3). The same significant difference was observed in the subgroup of patients with nicotine abuse (P<0.001), while the minimal number of non-smoking patients (N=10) does not permit any safe conclusions to be drawn. Finally, there were no major differences in the frequencies of the two IL-6 alleles due to categorizations of sex, age, and age at onset of oral cancer. Interestingly, compared to individuals with the GG genotype, the relative risk for oral squamous cell carcinoma for GC heterozygotes was 3.74 (2.29-6.11), while for CC homozygotes was 7.39 (2.61-20.62). Additionally, GC heterozygotes have a relative risk of 2.81 (1.56-4.96) for developing oral cancer in stages I&II and 5.6 (2.82-11.13) in stages III&IV. For CC homozygotes the respective relative risks are even greater, 5.94 (1.89-18.64) for stages I&II and 10.82 (2.93-39.94) for stages III&IV. 7 Discussion IL-6 is a multifunctional molecule produced by a variety of cells (12). The cytokine exerts its effects through activating pathways mediating cell proliferation and by inducing proteins that inhibit apoptosis (12). In neoplastic disease, IL-6 circulating levels increase markedly during development and progression of tumors (12). Indeed, high IL-6 levels have been shown to correlate with poor prognosis and high mortality in prostate and colorectal cancer patients (9-11). A single nucleotide polymorphism was identified at position -174 within the promoter of the IL-6 gene with two alleles, G and C. IL-6 gene expression is constitutively greater when the more common G allele is present, while it is elevated by chronic inflammation in the presence of the C allele (14,16-17). Furthermore, several studies in oral cancer, report elevated IL-6 in serum, saliva and tumor biopsies obtained from patients with oral cancer (18-19). In light of the above, in the present study the genotypes and allele frequencies of the -174G>C polymorphism were investigated in a cohort of 162 patients with oral cancer in comparison to 156 healthy controls of equivalent age, sex and ethnicity. Despite the relatively small sample of studied individuals, the overall obtained data revealed a strong association of the -174G>C IL-6 polymorphism with an increased risk for oral squamous cell carcinoma. Compared to the controls, a significant increase of GC heterozygotes and CC homozygotes was observed not only in the whole group of patients, but in all subgroups of patients regardless their alcohol consumption habits, early or advanced stage of cancer, presence or absence of a family history for cancer or 8 thrombophilia. Accordingly, the G allele frequency was decreased in the whole group of patients and in every subgroup. IL-6 gene expression is constitutively greater when the more common G allele is present, and this allele has been incriminated for increased risk of thrombotic events (14,16). In light of the above, the fact that the G allele frequency is decreased in the whole group of patients with oral cancer, as well as in every subgroup of them, might indicate that C allele carriers are protected from immediate risk for life-threatening thrombotic events, but not from cancer, which is a more prolonged process of multiple steps. This notion is in accordance not only to the findings of the present study, but with reports of association of the C allele with increased risk for breast and colorectal cancer (14,15). Furthermore, the fact that the C allele frequency is increased in the whole group and in every subgroup of our patients might suggest that it is actually the presence of this allele that causes the elevation in IL-6 circulating levels reported in several studies investigating serum, saliva and biopsies of patients with oral cancer (18,19). This notion is consistent with the C allele-related increase of IL-6 gene transcription observed in elderly individuals (over 40 years-old), such as those studied in the present work (17). It follows that, in C allele carriers age-related inflammation may increase risk for oncogenesis in certain tissues (including those in the oral region), while the actual target tissue may depend on accidental mutational events or environmental factors such as tobacco abuse. Unfortunately, the small amount of available non-smoking patients who were included in this study did not allow us to further test this hypothesis. 9 Additionally, the statistical analysis showed that CC homozygotes have twice as much greater relative risk for developing oral cancer than GC heterozygotes. Furthermore, C allele carriers have twice as much greater relative risk for developing oral cancer in stages III&IV than in I&II. Therefore, it can be assumed that -174G>C IL-6 promoter polymorphism is associated with a more aggressive type of oral cancer. In conclusion, the studied IL-6 polymorphism is strongly associated with increased risk for oral cancer in certain individuals. Similar associations have been reported for polymorphisms in other interleukin genes, as well (20). As a consequence, it is of great importance to perform further genetic association studies regarding the contribution of additional cytokines or other factors related to angiogenesis, inflammation and thrombosis to oncogenesis in the oral region. Any positive findings could ultimately result in the undertaking of preventive measures safeguarding the health status and lives of certain at risk individuals in the general population. 10 References 1. Silverman S Jr. Demographics and occurrence of oral and pharyngeal cancers. The outcomes, the trends, the challenge. J Am Dent Assoc 2001;132:7S–11S. 2. Williams HK. Molecular pathogenesis of oral carcinoma. J Clin Pathol 2000;53:165-72. 3. Song C, Xing D, Tan W, Wei Q, Lin D. Methylenetetrahydrofolate reductase polymorphisms increase risk of esophageal squamous cell carcinoma in a Chinese population. Cancer Res 2001;61:3272-5. 4. Vairaktaris E, Yapijakis C, Wiltfang J et al. Are factor V and prothrombin mutations associated with increased risk of oral cancer? Anticancer Res 2005; 25: 2561-6. 5. Vairaktaris E, Yapijakis C, Vylliotis A et al. Methylenetetrahydrofolate reductase polymorphism and minor increase of risk for oral cancer. J Cancer Res Clin Oncol 2006;132(4):219-22. 6. Vairaktaris E, Yapijakis C, Serefoglou Z et al. Plasminogen activator inhibitor-1 polymorphism is associated with increased risk for oral cancer. Oral Oncol (in press). 7. Vairaktaris E, Yapijakis C, Derka S et al. Association of platelet Ia polymorphism with minor increase of risk for oral cancer. Eur J Surg Oncol 2006;32(4):455-7. 8. Humphries S.E, Luong L.A, Ogg M.S, Hawe E, Miller G.J. The interleukin-62174 G/C promoter polymorphism is associated with risk of coronary heart disease and systolic blood pressure in healthy men. Eur. Heart J 2001;22:2243–52. 9. Srivani R, Nagarajan B: A prognostic insight on in vivo expression of interleukin-6 in uterine cervical cancer. Int J Gynecol Cancer 2003;13:331-9. 10. Chung YC, Chang YF: Serum interleukin-6 levels reflect the disease status of colorectal cancer. J Surg Oncol 2003;83:222-6. 11 11. Michalaki V, Syrigos K, Charles P, Waxman J: Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer 2004;90:2312-6. 12. Trikha M, Corringham R, Klein B, Rossi JF: Targeted antiinterleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res 2003;9:4653–65. 13. Bruunsgaard H, Christiansen L, Pedersen AN, Schroll M, Jorgensen T, Pedersen BK. The IL-6 -174 G>C polymorphism is associated with cardiovascular diseases and mortality in 80-year-old humans. Exp Gerontol 2004;39:255-61. 14. DeMichele A, Martin AM, Mick R et al. Interleukin-6 -174 G>C polymorphism is associated with improved outcome in high-risk breast cancer. Cancer Res 2003;63:8051-6. 15. Landi S, Moreno V, Gioia-Patricola L et al. Association of common polymorphisms in inflammatory genes interleukin(IL)6, IL8, tumor necrosis factor α, NFKB1, and peroxisome proliferators-activated receptor γ with colorectal cancer. Cancer Res 2003;63:3560-6. 16. Belluco C, Olivieri F, Bonafe M et al. -174 G>C polymorphism of interleukin 6 gene promoter affects interleukin 6 serum levels in patients with colorectal cancer. Clin Cancer Res 2003;9:2173-6. 17. Berger FG. The interleukin-6 gene: a susceptibility factor that may contribute to racial and ethnic disparities in breast cancer mortality. Breast Cancer Res Treatment 2004;88:281-5. 12 18. Wang YF, Chang SY, Tai SK, Li WY, Wang LS. Clinical significance of interleukin- 6 and interleukin-6 receptor expressions in oral squamous cell carcinoma. Head Neck 2002;24(9):850-8. 19. Vucicevic BV, Cikes N, Lukac J, Virag M, Cekic-Arambasin A. Salivary and serum interleukin 6 and basic fibroblast growth factor levels in patients with oral squamous cell carcinoma. Min Stomatol 2005;54(10):569-73. 20. Tsai MH, Chen WC, Tsai CH, Hang LW, Tsai FJ. Interleukin-4 gene, but not the interleukin-1 beta gene polymorphism, is associated with oral cancer. J Clin Lab Anal. 2005;19(3):93-8. 13 Table 1. Prevalence of IL-6 (-174G/C) polymorphism in healthy controls and patients with oral cancer (total group of patients and subgroups in regard to cancer stage). aFischer’s P-value; bχ2 P-value; cage-adjusted odds ratios. Genotypes C/C G/G G/C Total Prevalence of C allele C allele frequency Carrier frequency of C allele Controls (%) Patients (%) P-value ORc (CI) 6 (3.8%) 90 (57.7%) 60 (38.5%) 156 (100%) 18 (11.1%) 42 (25.9%) 102 (63.0%) 162 (100%) P<0.001a 7.39 (2.61 - 20.92) 72 (23.1%) 138 (42.6%) P<0.001b 72 (40%) P<0.001b 66 (45.8%) P<0.001b 66 (42.3%) 120 (74.1%) P<0.001b 62 (68.9%) P<0.001b 58 (80.6%) P<0.001b 1 (referent) P<0.001a 3.74 (2.29 – 6.11) Patients with cancer stages I&II (%) 10 (11.1%) 28 (31.1%) 52 (57.8%) 90 (100%) P-value ORc (CI) P<0.001a 5.94 (1.89-18.64) 1 (referent) P<0.001a 2.81 (1.56-4.96) Patients with cancer stages III&IV (%) 8 (11.1%) 14 (19.4%) 50 (69.5%) 72 (100%) P-value ORc (CI) P<0.001a 10.82 (2.93-39.94) 1 (referent) P<0.001a 5.6 (2.82-11.13) 14 Table 2. Prevalence of IL-6 (-174G/C) polymorphism in healthy controls and patients with oral cancer in regard to family history of either cancer or trombophilia. aFischer’s P-value; bχ2 P-value; cage-adjusted odds ratios. Genotypes C/C G/G G/C Total Prevalence of C allele C allele frequency Carrier frequency of C allele P-value ORc (CI) 6 (3.9%) 90 (57.7%) 60 (38.5%) 156 (100%) Patients with family history of cancer (%) 6 (10%) 12 (20%) 42 (70%) 60 (100%) P<0.001a 7.19 (2.03-25.47 72 (23.1%) 54 (45%) 66 (42.3%) 48 (80%) Controls (%) Patients without family history of cancer (%) 12 (11.8%) 30 (29.4%) 60 (58.8%) 102 (100%) P-value ORc (CI) P<0.001a 7.47 (2.4-23.2) P<0.001b 84 (41.2%) P<0.001b 72 (70.6%) 1 (referent) P<0.001a 5.23 (2.54-10.74) Patients with family history of thr (%) 8 (25%) 8 (25%) 16 (50%) 32 (100%) P-value ORc (CI) P<0.001a 13.02 (3.74-45.34) P<0.001b 32 (50%) P<0.001b 24 (75%) 1 (referent) P<0.001a 3.11 (1.78-5.43) Patients without family history of thr (%) 10 (7.7%) 34 (26.2%) 86 (66.1%) 130 (100%) P-value ORc (CI) P<0.001a 5.49 (1.74-17.31) P<0.001b 106 (40.8%) P<0.001b P<0.001b 96 (73.8%) P<0.001b 1 (referent) P<0.001a 2.95 (1.2-7.29) 1 (referent) P<0.001a 3.98 (2.36-6.71) 15 Table 3. Prevalence of IL-6 (-174G/C) polymorphism in healthy controls and patients with oral cancer in regard to either alcohol consumption or smoking habits. aFischer’s P-value; bχ2 P-value; cage-adjusted odds ratios; N.S.: not significant P-value. Genotypes C/C G/G G/C Total Prevalence of C allele C allele frequency Carrier frequency of C allele P-value ORc (CI) Patients without tobacco abuse (%) 18 (11.8%) 38 (25%) 96 (63.2%) 152 (100%) P<0.001a 8.87 (3.06-25.68) 0 (0%) 4 (40%) 6 (60%) 10 (100%) P<0.001a 72 (23.1%) 132 (43.4%) P<0.001b 6 (30%) P<0.001b 44 (42.3%) N.S. 94 (42.7%) P<0.001b 66 (42.3%) 114 (75%) P<0.001b 6 (60%) N.S. 38 (73.1%) P<0.001b 82 (74.5%) P<0.001b Controls (%) Patients with tobacco abuse (%) 6 (3.9%) 90 (57.7%) 60 (38.5%) 156 (100%) 1 (referent) P<0.001a 3.98 (2.4-6.6) P-value ORc (CI) 1 (referent) P<0.001a 2.14 (0.6-7.65) Patients with alcohol abuse (%) 6 (11.5%) 14 (26.9%) 32 (61.6%) 52 (100%) P-value ORc (CI) P<0.001a 9.31 (2.27-38.11) 1 (referent) P<0.001a 3.68 (1.78-7.59) Patients without alcohol abuse (%) 12 (10.9%) 28 (25.5%) 70 (63.6%) 110 (100%) P-value ORc (CI) P<0.001a 6.75 (2.23-20.45) 1 (referent) P<0.001a 3.76 (2.17-6.5) 16 Figure 1: Nla III restriction pattern of the IL-6 -174 G>C polymorphism, observed in three patients (CC homozygote , GC heterozygote, GG homozygote, M: molecular weight marker). M GC GG GG CC 93 bp 52/41 bp 17