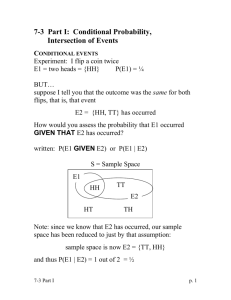
Appendix S3 Detection probabilities
advertisement

Appendix S3 Detection probabilities The matrix of presence / absence of the 71 species over the ten point counts of each square was processed for 2003 and 2004 to estimate mean detection probabilities (1) in each square for the global community and (2) across squares for each individual species, using probabilistic capture-recapture models for closed animal populations (Burnham & Overton 1979; Pollock 1982). The application of closed population models is straightforward (Boulinier et al. 1998) because it is reasonable to think that a sampled community is closed to local extinction and colonisation for the short period over which the species presence-absence data are collected. Estimates of detection probabilities were obtained by running the program COMDYN (Hines et al. 1999). The algorithm of COMDYN allows us to consider heterogeneous detection probability among raw data (here either species within a square’s community, or squares within individual species’ data), using the capture-recapture model M(h) and the associated jackknife estimator (Burnham & Overton 1979). This model is the most frequently selected model in the framework of species richness estimation when using breeding bird survey data (Boulinier et al. 1998; Alpiza-Jara et al. 2004; Jiguet et al. 2005). Mean detection probabilities were estimated (1) for each square for the 71 species’ community in 2003 and 2004 (one mean value for the community per square for each year), and (2) for each species across all squares in 2003 or 2004 (one mean value per species for each year). We then compared detection probability between years across the 653 sites for (1) and across the 71 species for (2) using paired t-tests to evaluate whether it varied among years. We also tested the relationship between thermal range and the difference in individual species’ detection probability between 2003 and 2004 using Pearson’s correlation coefficient. Finally, we estimated mean detection probabilities of individual species in the hottest and coolest squares surveyed in 2003 (using spring and summer temperature anomalies, respectively, to identify hottest and coolest squares), and tested the relationship between thermal range and the difference in individual species’ detection probability between hot and cool sites, using Pearson’s correlation coefficient. We found no variation in mean detection probabilities of the 71 species’ community for all monitored sites between 2003 and 2004 (paired t-test for sites, t652 = -0.43, P = 0.67). There was also no variation in mean detection probabilities for the 71 species between 2003 and 2004 (paired t-test for species, t70 = 0.47, P = 0.64), and variation in mean detection probabilities in 2003 and in 2004 for the 71 species was not correlated with the thermal range (n = 71, r = -0.02, P = 0.86). The further comparison of 2003 mean detection probability between the hottest (with the largest temperature anomalies in 2003) and the coldest (with the lowest temperature anomalies in 2003) surveyed squares for species with enough available data again revealed no significant trend between differences in detection probability (between hottest and coolest squares in 2003) and thermal range (Pearson’s correlation coefficients; spring temperature anomalies: n = 68, r = 0.06, P = 0.60; summer temperature anomalies: n = 67, r = -0.10, P = 0.41). So we found no variation in detection probabilities between 2003 and 2004, when considering either the surveyed sites (mean detection probability of the 71 species within the community) or the studied species (mean detection probability of individual species across sites). Moreover, variation in detection probability of individual species between 2003 and 2004 was not related to the thermal range of the species concerned, and variation in detection probability of individual species between the hottest and coolest (either in spring or summer) sites surveyed in 2003 was not related to the species’ thermal range. Therefore, we conclude that the correlation between thermal range and response of growth rate to temperature anomaly is not an artefact of variation in species’ detection under different climatic conditions. REFERENCES Alpiza-Jara, R., Nichols, J.D., Hines, J.E., Sauer, J.R., Pollock, K.H. & Rosenberry, C.S. (2004). The relationship between species detection probability and local extinction probability. Oecologia, 141, 652-660. Boulinier, T., Nichols, J.D., Sauer, J.R., Hines, J.E., & Pollock, K.H. (1998). Estimating species richness: the importance of heterogeneity in species detectability. Ecology, 79, 1018-1028. Burnham, K.P. & Overton, W.S. (1979). Robust estimation of population size when capture probabilities vary among animals. Ecology, 60, 927-936. Hines, J.E., Boulinier, T., Nichols, J.D., Sauer, J.R., & Pollock, K.H. (1999). COMDYN: software to study the dynamics of animal communities using a capture-recapture approach. Bird Study, 46, 209217. Jiguet, F., Renault, O. & Petiau, A. (2005) Estimating species richness with capture-recapture models in heterogeneous conditions: choice of model when sampling in heterogeneous conditions. Bird Study, 52, 180-187. Pollock, K.H. (1982). A capture-recapture design robust to unequal probability of capture. J. Wildlife Manage., 46, 752-757.