EXAM REVIEW jan2015
advertisement

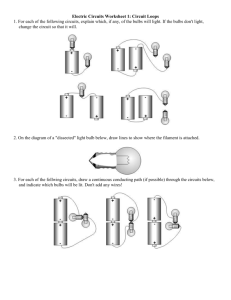
SNC 1D ECOLOGY REVIEW 1. Define the following: Ecosystem, biodiversity, herbivore, omnivore, carnivore, scavenger, decomposer, pyramid of energy, predator-prey, bioaccumulation (bioamplification), niche, habitat 2. List the following terms in order from smallest to largest: biome, ecosystem, biosphere, habitat, community, individual, population 3. Name the biomes in Canada. What are they like? 4. Identify the following limiting factors as either abiotic or biotic. a. Wind blows the seeds of a dandelion into a pond. The seeds fail to grow. b. A population of grasshoppers eats all the available food and their numbers drop. c. A bacterium causes a deadly disease in a herd of reindeer and some of them die. d. Plants growing beneath the trees in a forest are unable to get enough sunlight. 5. List the instruments needed for measuring abiotic factors in an ecosystem. 6. Explain why the base of the food chain must be a producer 7. Construct a food chain of 4 individuals from a terrestrial ecosystem. 8. Identify the trophic levels for each individual in the food chain. 9. Explain the difference between a food web and a food chain. 10. What does an energy (or food) pyramid represent? 11. Why is the base of a pyramid of energy so wide compared to its height? 12. Make a pyramid of energy with 4 trophic levels. Label the diagram: producer, autotroph, heterotroph, primary consumer, secondary consumer, tertiary consumer, top carnivore. 13. What is the original source of energy for all organisms in the pyramid of energy? 14. Consider a situation where a squirrel eats a nut. a. How much of the nut’s energy will be incorporated into the squirrel’s tissues? b. Explain what happens to the remaining energy. 15. A food web contains plants, grasshoppers, frogs, snakes, insect-eating birds, and falcons. a. Identify the group that contains the most energy. b. Rank the remaining groups, from most to least in terms of energy content. 16. Draw and label the water cycle and the carbon cycle. 17. What are two processes that cause carbon to enter the atmosphere? 18. Photosynthesis and cellular respiration are called “complementary processes.” explain 19. Classify the following interactions as mutualism, commensalism, or parasitism. a. A yucca moth caterpillar feeds on the yucca plant and pollinates the yucca plant. b. Lice feed harmlessly on the feathers of birds. c. A cowbird removes an egg from a robin’s nest and replaces it with its own. d. An orchid plant grows on the branch of a tree. The tree remains healthy. 20. Bacteria can reproduce so quickly that, under ideal conditions, one bacterium could produce enough bacteria to cover the entire planet in only a few weeks. Explain why this does not happen. 21. Suppose that a herd of reindeer had been introduced to a small island that had no reindeer on it, and their population had been monitored. The table below shows the population data. Reindeer Population 1910-1945 Year Population 1910 50 1920 250 1930 500 1935 2000 1940 1400 1945 1500 a. Graph the population data. Don’t forget the title and label the axes. b. Label the parts of the graph. (slow growth, population start-up, exponential growth, steady state (carrying capacity), exponential growth) c. What is the carrying capacity of the reindeer? Show this on the graph. d. Suggest why the population of reindeer was lower in 1940 than in 1935. 22. Estimate the population size using the following samples taken from a 1000quadrat field. 7,17,5,3,12,15,21,0,6 23. Easter Island (pg.52~53) represents an unsustainable ecosystem. You have learned that five factors can affect the sustainability of ecosystems. Did all of these factors contribute to the islanders’ demise? Justify your answer. SNC 1D1 EXAM REVIEW: Chemistry 1. Study the safety symbols so that you know what each one means. 2. (a) What is a physical property of matter? (b) Give six examples. 3. (a) What is a chemical property of matter? (b) Give two examples. 4. (a) What is density? (b) Write the formula for density. (c) Calculate the density of a substance that has a mass of 200 g and a volume of 40 cm3. (d) Would the substance sink or float on water? (e) How do you know what the substance is? 5. (a) What is a physical change? (b) Identify the six changes of state by their scientific names. (c) Name the change of state that has occurred when (i) moth balls disappear (ii) frost forms on a window (iii) a mirror fogs up (iv) a chocolate bar gets soft in your hand. 6. (a) What is a chemical change? (b) Give five pieces of evidence (clues). 7. Classify each of the following as either a physical change or a chemical change. (a) garbage rotting (b) cutting up carrots (c) a silver spoon turns black (d) bleaching a stain (e) alcohol evaporating (f) breaking glass (g) burning magnesium (h) a copper roof turns green 8. State the four principles of Particle Theory. 9. Define and give two examples of: (a) element (b) compound (c) pure substance (d) solution (e) heterogeneous mixture (f) alloy. 10. How would you test to see if a gas was (a) hydrogen, (b) oxygen, (c) carbon dioxide? 11. What is a (a) Group (b) Period? 12. Where are each of the following found on the periodic table (a) metals (b) non-metals (c) alkali metals (d) alkaline earth metals (e) halogens (f) noble gases (g) metalloids? 13. Give the typical properties of a (a) metal (b) non-metal. 14. Identify the three subatomic particles by name, charge, mass and location in the atom. 15. Define (a) atomic number (b) mass number 16. Identify the number of protons, neutrons and electrons in (a) C (b) oxygen (c) Ca 17. Write the standard atomic notation for (a) atom of sulfur (b) an atom with 3P, 3e and 4N. 18. Draw a Bohr-Rutherford diagram for (a) fluorine (b) sodium (c) magnesium. 19. Without drawing a Bohr-Rutherford diagram, how could you quickly tell (a) how many electrons were in the outer energy level of an atom? (b) the number of energy levels in an atom? 20. State how many different elements, the number of atoms of each element and the total number of atoms for each of the following (a) NaHCO3 (b) NaOH (c) NaNO3 (d) C9H 8O4 21. Name the following compounds (a) MgCl2 (b) Na2S (c) K2O (d) NaF (e) Li3N SNC 1D1 EXAM REVIEW: Electricity 1. Define each of the following terms: (a) static electricity (b) conductor (c) insulator. 2. Describe charging by (a) friction p274 (b) contact p278 (c) induction p285. 3. How does an object become (a) negatively charged (b) positively charged? 4. State the 3 Laws of Electric Charge. 5. (a) Use the Triboelectric series to predict the charge on a piece of rubber if it is rubbed with wool (b) Predict the charge on a piece of glass if it is rubbed with silk. 6. Using a diagram and several sentences, explain what happens on a dry, cool day if you drag your feet across a carpet and then reach out and touch a metal doorknob. 7. Identify 3 useful and 3 harmful examples of static. 8. Using a diagram and several sentences, explain how a lightning rod works. 9. What are the 4 parts of a circuit? Describe them and give examples. 10. (a) What is a series circuit? (b) What is a parallel circuit? (c) Draw a circuit diagram showing three cells in series connected to two bulbs in series. Include a switch and a voltmeter on one bulb. (d) Draw a circuit diagram showing two cells in series connected to two bulbs in parallel. Include a switch that will control both bulbs. 11. For each of electric potential (voltage) and electric current (a) Define the terms (b) Give their symbols (c) State how they are measured. (d) Give the name/symbol of the units that are measured. 12. In a series circuit consisting of one cell and 2 bulbs, (a) how does the current between the bulbs compare to the current immediately after the cell? (b) How does the voltage drop at one of the bulbs compare to the voltage measured across the cell? (c) What happens if you remove one of the bulbs from its holder? 13. In a parallel circuit consisting of one cell and 2 bulbs, (a) how does the current through each of the bulbs compare to the current immediately after the cell? (b) How does the voltage drop at one of the bulbs compare to the voltage measured across the cell? (c) What happens if you remove one of the bulbs from its holder? 14. (a) What are the parts of a wet cell? (b) What is the difference between a cell and a battery? (c) What is the difference between a primary and a secondary cell? 15. (a) Define electrical resistance, give its symbol and state its units (b) Write the formula for Ohm’s Law (c) Calculate the resistance in a toaster that operates on 120V and uses 15A of current. (d) Find the voltage required to force a current flow of 2 A when the resistance is 100Ω. (e) Determine the current flowing from a 3 V battery if its resistance is 30Ω. 16. Why are circuit breakers and fuses considered to be safety devices? SNC 1D1 EXAM REVIEW: SPACE 1. Outline the lifecycle of a star. 2. What is GPS, ISS, AU, light year? 3. Describe 3 real risks or hazards associated with space travel and exploration. 4. Give 3 examples of a spinoff from space-related research or technology that now has a use here in our lives on Earth. 5. Why do we have orbiting satellites? 6. Explain the difference between rotation and revolution.