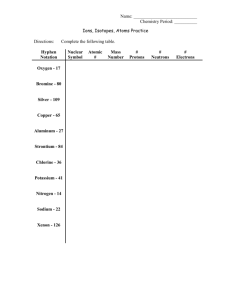
Elements that form ions having
advertisement

Stock System for elements that form ions having more than one charge Name______________________________ Date________ Block______ Most of the transition metals and some of the metals in groups 13, 14, 15, and 16 can form ions with more than one charge. Only zinc and silver have fixed charges. This variation in charge occurs because the transition metal atoms may use one or more of their d sublevel electrons as a valence electron, or, as in the case of metals in groups 13-16, sometimes, only the p sublevel valence electrons are lost. Naming these ions is easy. The Stock system uses the name of the element and a Roman numeral representing the size of the charge that is written after the name of the element. Note: the Roman numeral is NOT used when writing formulas involving these metals. The following are examples of metals that form ions having more than one charge. Only the more common charges have been given here (some may form ions having other charges). Fe+2 Fe+3 iron(II) ion iron(III) ion Hg2+2 mercury(I) ion* Hg+2 mercury(II) ion Sn+2 tin(II) ion Sn+4 tin(IV) ion Cu+1 Cu+2 copper(I) ion copper(II) ion Pb+2 Pb+4 lead(II) ion lead(IV) ion Cr+2 Cr+3 chromium(II) ion chromium(III) ion Mn+2 Mn+3 manganese(II) ion manganese(III) ion Co+2 Co+3 cobalt(II) ion cobalt(III) ion Cd+2 Cd+3 cadmium(II) ion cadmium(III) ion Ti+2 Ti+4 titanium(II) ion titanium(IV) ion Bi+3 Bi+5 bismuth(III) ion bismuth(V) ion * NOTE: the mercury(I) ion has two atoms covalently bonded that act as a single ion with a charge of +2 because each atom has lost one valence electron; the mercury(II) ion is a single mercury atom that has lost its 2 valence electrons and it has a charge of +2. Naming Compounds Containing Ions That Can Have More Than One Charge: To name such compounds, simply follow the rule for naming binary ionic compounds: name the cation first, then name the anion. The only difference is that you MUST include the Roman numeral for the charge of the cation. Remember that the anions have fixed charges. Examples: In the formula MnO, the manganese ion has a +2 charge and the oxide has –2 charge. The compound is called manganese(II) oxide. In the formula CuS, the copper ion has a charge of +2 (there is only one copper ion and there is one sulfide ion with a charge of –2, so the copper ion must have a charge of +2). The compound is named copper(II) sulfide. In the formula Cu2S, there are two copper ions and one sulfide ion. Each of the copper ions must have a charge of +1 to give a total positive charge of +2 to balance the total negative charge of –2. Therefore, the compound is called copper(I) sulfide. To write the formula when given the name of a compound, write the symbol for each ion above its name first. Then write a balanced formula. Cu+2 S-2 copper(II) sulfide Fe+3 O-2 iron(III) oxide CuS Fe2O3 Cu+1 S-2 copper(I) sulfide Cu2S Fe+2 O-2 iron(II) oxide FeO Remember: Roman numerals are NOT written in the chemical formula! For each of the following, write the correct chemical formula: 1. copper(I) oxide _____________ 11. iron(III) oxide ____________ 2. lead(II) chloride ____________ 12. lead(IV) oxide ____________ 3. cobalt(II) chloride ___________ 13. cobalt(III) chloride __________ 4. mercury(II) bromide __________ 14. mercury(I) bromide __________ 5. mercury(I) oxide __________ 15. mercury(II) oxide __________ 6. chromium(III) nitride __________ 16. chromium(II) nitride _________ 7. tin(IV) fluoride __________ 17. iron(II) sulfide _________ 8. iron(III) oxide ___________ 18. cadmium(II) bromide _________ 9. tin(IV) oxide ___________ 19. manganese(II) fluoride __________ 10. manganese(III) chloride __________ 20. mercury(I) nitride __________ Write the correct names for each of the following: 1. MnO _____________________________________ 2. PbO2 _____________________________________ 3. Fe2O3 ____________________________________ 4. Cu3P2 _____________________________________ 5. SnF2 _____________________________________ 6. CuCl _____________________________________ 7. PbI2 ______________________________________ 8. SnCl4 _____________________________________ 9. CrO3 _____________________________________ 10. MnO ___________________________________ 11. TiO2 ___________________________________ 12. BiF5 ______________________________________ 13. PbCl4 _____________________________________ 14. CrF3 _____________________________________ 15. NiBr2 ____________________________________ 16. CrO2 ___________________________________ 17. Mn2O7 ___________________________________ 18. Fe2O3 ___________________________________ 19. NiO ____________________________________ 20. Mn2O5 ___________________________________