Investigation_3_3_from_campbell_cd
advertisement

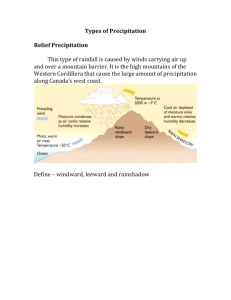
Text from Investigation 3.3 from Campbell CD This activity introduces some of the processes of science— making observations, asking questions, gathering information, posing hypotheses, testing hypotheses experimentally, and reaching conclusions— through a study of acid precipitation.Acid precipitation (rain, snow, fog) has an especially high concentration of hydrogen ions. In other words, it is acidic. Resulting mainly from the burning of fossil fuels (coal, oil, and gas), acid precipitation is a major environmental problem because it can kill aquatic organisms, destroy forests, and damage buildings and monuments. Click on the Questions window and answer Question 1. Imagine that you are traveling through the mountains of the eastern U.S. You notice large areas of forest that are dead or dying. Later, you tell your biology instructor about the trip, and she suggests that you may have seen forests affected by acid precipitation.However, acid rain may not be the only pollutant killing the trees. Consider other hypotheses by answering Question 2. You decide to pursue the research project on acid precipitation. To begin gathering background information about acid precipitation, click on "What is an acid?" A chemical compound that dissociates (breaks up) and forms hydrogen ions (H+ ions) in a solution is called an acid. The more acidic the solution, the higher its concentration of H+ ions. Sulfuric acid (H2SO4), one of the major acids found in acid rain, dissociates in water to form H+ and HSO4- ions, thus liberating H+ ions in the water solution. Answer Question 3. Then click on "What is pH?" The pH scale measures the degree of acidity, from 0 to 14. Those substances with a pH of less than 7 are considered acids. The higher the H+ concentration, the stronger the acidity, and the lower the pH. Answer Question 4. Then click on "What are the major sources of acid precipitation?" Acid precipitation results mainly from sulfur dioxide and nitrogen oxides that react with water in the air to form sulfuric and nitric acids. These acids fall to the ground in the form of rain, snow, or fog. Sulfur dioxide and nitrogen oxides are mainly the result of fossil fuel burning in coal-burning electric power plants (the single largest source of acid pollutants), factories, and automobiles. Answer Question 5. Then click on "What are some of the harmful effects of acid precipitation?" Strong acidity can break down the molecules of living organisms. Even if molecules remain intact, they may not be able to perform their normal, essential chemical processes. Thus, many different functions of organisms may be affected by acid precipitation. Strong acidity can kill aquatic organisms, destroy forests, and damage buildings and monuments. Use this information to answer Question 6. Then click on "Where is acid precipitation found in the United States?" These maps show the average pH of annual precipitation in the U.S. in 1955 and 1990. Click on the button to compare maps. The numbers next to the black lines and the color code refer to pH. Precipitation was more acidic in the colored areas than in the black and gray areas. Precipitation was most acidic in the lightest green areas. Note how in some areas acidity increased (pH has decreased) dramatically. Use this observation to answer Question 7. Then click on "Which areas in North America are sensitive to acid precipitation?" The black dots on this map indicate the location of the major human sources of pollutants associated with acid precipitation. The green portions of the map indicate areas in the U.S. and Canada that are especially sensitive to acid precipitation (that is, the soil and water in these areas have little ability to effectively buffer or neutralize acids). Use this information to answer Question 8. Then click Next. The scientific method is fundamentally a way of answering questions. To proceed with your acid precipitation research, you need to focus on a particular question. Which of these questions follows most logically from your observations and from the background information you have gathered? Before investigating your research question, you need a bit more information. On a map showing the annual average pH of precipitation in areas across the U.S., you add red and blue dots— assigning red dots to sites with dying forests and blue dots to sites with healthy forests.How can you best use this map (before you test a specific hypothesis) to obtain additional evidence that acid precipitation may have caused the forest destruction you observed? Click on the map to see the choices. Then click on your choice. It is difficult to test directly a hypothesis that proposes an explanation for such a large-scale phenomenon as acid precipitation. Researchers often test different aspects relating to a large-scale hypothesis to support or disprove the hypothesis. For example, acid precipitation may affect trees in several ways, as shown here.Let's focus on leaf damage. Click on the image to see two hypotheses. Which one would be the most reasonable to test? Click on your choice. f a hypothesis is correct and you perform a test of the hypothesis, then you can expect a particular outcome. A prediction states the results you would expect if a hypothesis is correct. Which of these predictions would best test the hypothesis that acid precipitation damages the leaves of young (oneyear-old) red oak trees? Now you can set up an experiment to test your hypothesis. You start by placing 100 one-year-old red oaks in one-gallon pots, with equal amounts of soil in each. Once a month for a year, you will measure the total number of leaves and the percentage of damaged leaves found on each tree. Which of the following experiments would be most appropriate to test the hypothesis that acid precipitation damages the leaves of young red oak trees? (1) You water each tree daily with 100 ml of water, giving all 100 trees acidic water with a pH of 4.5. (2) You water each tree daily with 100 ml of water, giving 50 trees acidic water with a pH of 4.5 and 50 trees "normal" water with a pH of 5.6. (3) You water each tree daily with 100 ml of water, giving 50 trees acidic water with a pH of 4.5 and 50 trees acidic water with a pH of 5. Click on what you think is the best experiment. Now you proceed with your experiment, giving the control trees water similar to normal rainwater and the experimental trees water with the pH of acid precipitation. Once a month you count and record the number of leaves found on the trees, and the percentage of damaged leaves, for the experimental and the control plants. Then you calculate average numbers and percentages for each treatment. These graphs summarize your data. Study the graphs and answer Question 9. Then state your conclusion by answering Question 10. Investigation 3.3 from Campbell CD Name: _______________________ Period: ____ 1. What are probable sources of acid precipitation in your community? What impact can this have on the environment in your region? 2. What factors apart from acid rain could be killing trees? 3. Limestone, an underlying material for many soils, dissolves and corrodes when exposed to high concentrations of hydrogen ions. How is this information important in understanding the effects of acid precipitation? 4. Acid precipitation is considered severe if it falls below pH 4. Considering the pH of rainwater, how easy would it be to convert the rainwater to acid precipitation? 5. Carbon dioxide forms a weak acid called carbonic acid when dissolved in water. How can this be a significant factor in the formation of acid rain? 6. Frogs are very sensitive to acid rain. How could this information be used to investigate your observation about the trees dying in the forest? 7. What events could have created the increase in acid precipitation between 1955 and 1990? 8. Why is the Canadian government particularly concerned about negotiating clean air agreements with the United States? 9. What is the average number of leaves per tree and the percentage of damaged leaves for both the experimental and control plants over the duration of the experiment? 10 . What can you conclude from these results?