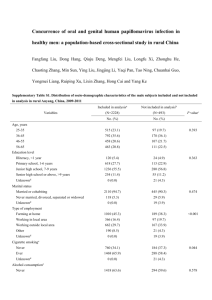
Curriculum Vitae - Georgetown University
advertisement

Richard Schlegel, M.D., Ph.D. Oscar B. Hunter Chair, Department of Pathology Director, Center for Cell Reprogramming Professor of Pathology, Oncology, Microbiology and Immunology, and Obstetrics and Gynecology Georgetown University Medical Center, Washington, DC Office Address: Department of Pathology Research Building, Rm W500 Georgetown University Medical School 3900 Reservoir Road, NW Washington, DC 20057 Telephone: 202-687-1655; FAX: 202-687-2933; e-mail: schleger@georgetown.edu Citizenship: USA Medical licensure: Massachusetts (registration number 40361; initial date of licensure: August 1, 1977; inactive. District of Columbia (license No. 20823, Class 3NB; initial date of issue: July 15, 1994; expiration date: current) Certification: Diplomat: National Board of Medical Examiners American Board of Pathology Board Certification: Anatomic Pathology, 1979 Education: 1964-1968 1968-1975 1975-1979 1975-1980 1980-1982 B.A. Rutgers University, New Brunswick, New Jersey. Major in Biological Sciences. M.D./Ph.D., Northwestern University Medical School, Chicago, Illinois. Ph.D. in Microbiology mentored by Dr. Hutton Slade. Resident in Pathology, Harvard Medical School, Peter Bent Brigham Hospital, Boston, Massachusetts. Chairman: Dr. Ramzi Cotran. Research Fellow, Department of Pathology, Harvard Medical School, Boston, Massachusetts. Postdoctoral fellowship mentored by Dr. Thomas Benjamin. Staff Associate, Laboratory of Molecular Biology, National Cancer Institute, NIH. Mentored by Dr. Ira Pastan. Professional Experience: 1982-1984 1984-1990 1988-1990 1990-1992 1992- 1997-2002 2002- Senior Investigator, Laboratory of Pathology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland. Laboratory Chief: Dr. Lance Liotta. Chief, Cell Regulation and Transformation Section, Laboratory of Tumor Virus Biology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland. Laboratory Chief: Dr. Peter Howley. Clinical Associate Professor, Department of Pathology, Georgetown University School of Medicine, Washington, DC Acting Chairman: Dr. Bennett Jenson. Associate Professor, Department of Pathology, Georgetown University School of Medicine, Washington, DC Chairman: Dr. Jeffrey Cossman. Professor, Department of Pathology and Department of Obstetrics and Gynecology, Georgetown University School of Medicine. Interim Chair, Department of Pathology, Georgetown University School of Medicine Chair, Department of Pathology, Georgetown University School of Medicine Honors and Awards: Phi Beta Kappa, Rutgers University, 1967 Summa cum laude, Rutgers University, 1968 Henry Rutgers Honors Research Fellowship, 1967, 1968 Sigma Xi, First place, Graduate student research competition, Northwestern University, 1972. National Research Service Award, 5-T32-HL-07066-02, 1977 Public Health Service Commendation Award, 1987 Candidate for Bristol-Meyers Award for Distinguished Achievement in Cancer Research, 1992, 1993 Georgetown University Candidate for Howard Hughes Medical Investigators Award, 1993 Georgetown University Nominee for Bristol Myers Squibb Award for Distinguished Achievement in Infectious Disease Research, 1998 Georgetown University Nominee for Professor of the Year, 1998; Graduate Student Association of Georgetown Medical Center Award for Service Toward a Cure, Georgetown University and Lombardi Comprehensive Cancer Center, 2006 President's Medal, Georgetown University, November 2006 Vicennal Medal, Georgetown University, March 2010 Patrick Healy Award, Georgetown University Alumni Association, April 2011 Award from Office of Technology Commercialization, Georgetown University, “For outstanding Achievement in Innovation and Commercialization”, February 2011. President's Award for Distinguished Teacher-Scholars, induction into the Society of Presidential Fellows, Georgetown University, October 2013. National Academy of Inventors, February 2014. Special Research Recognition Award, Georgetown University, September 2014. Professional Societies: American Association for the Advancement of Science American Society of Microbiology American Association of Pathologists Editorial Boards: Virology, Associate Editor. (1990-2010) Reviewer: Science, Cell, EMBO J., Proc. Natl. Acad. Sci. USA, Nature Reviews, J.Virol., J. Exp. Med., Am. J. Path., Oncogene, Cancer Research, J. Cell. Physiol.., Anal. Biochem., Mol. Carcinogenesis, Clin. Microbiol., J. Nat. Cancer Inst. NIH Workshops: NCI Workshop on: The Transformation Mechanisms of Papillomaviruses, Feb. 18-19, 1986. (RFA development) (speaker) NCI Workshop on: Prospects for Human Papillomavirus Vaccines and Immunotherapies, Sep. 22, 1997. (RFA development) (speaker) NCI Cancer Vaccine Workshop (speaker) May Wong, DCB, NCI, November 4, 1998 NIH Review Committees: Site visit review committee, 2-PO1-CA30246-07, Regulatory functions of protein/ nucleic acid interactions, March 5, 1987. Special review committee, RFA 90-CA-09, New approaches to understanding transformation by SV40 Virus, Polyomaviruses, and Adenoviruses, November 4-5, 1990. Intramural Review Panel (Tenure and Promotion), Division of Cancer Prevention and Control, NCI (Ad Hoc Member) Chairman: Dr. Edward Sondik, Deputy Director, DCPC, Oct. 23, 1991. Special Review Committee, RFA-NIH-NIAID-92-AI-08, Papillomavirus in-vitro cell culture systems, March 3, 1993. Intramural Site Visit Review, Laboratory of Biology, National Cancer Institute, NIH, Chairperson: Dr. Noel Bouck, Northwestern University, September 25-27, 1996 Site visit review committee, 2P01CA22443-21, Molecular Biology and Genetics of Tumor Viruses, University of Wisconsin, Bill Sugden P.I., May 14-16, 1997. Pre-site visit advisory committee, P50DC00203, Papillomas from the upper respiratory tract, Dr. Betty Steinberg, June 4, 1997. Special Review Committee, RFA-CA-97-007, NCI Scholar’s Program, Rockville, MD, November 18, 1997. NCI Strategy-planning meeting on cervical cancer and HPV vaccines, Edward Trimble, DCTD, NCI, Peter Greenwald, DCP, NCI, Sheraton International Hotel, Baltimore-Washington International Airport, September 14-15, 1998 CRC (Cancer Research Campaign) Site Visit, Beatson Institute for Cancer Research, Glasgow, Scotland, Laboratories of Dr. Malcolm Finbow and Dr. John Pitts, February 4, 1999 Special review committee, Evaluation of the Applied Tumor Virology Program, German National Cancer Center, Heidelberg, Germany, June, 2001 NIH Virology study section , Ad hoc reviewer, Infectious Disease and Microbiology Integrated Review, Group, Bethesda, MD, Feb 26-27, 2002. NIH Experimental Virology study section, Ad hoc reviewer, Washington, DC, February 2003 NIH Experimental Virology study section, Ad hoc reviewer, Alexandria, VA, October 2003 NIH Virology B study section, Permanent member: November 2003 - October 2007 Scientific Advisory Boards: Pharmagenics, Inc., (1990-1993) Albert B. Sabin Vaccine Institute (1997-1999) Scientific Consultant: MedImmune, Inc. (1993-1996) Scientific Review Board: American Social Health Association (1995-2000) Scientific Advisor: Wyngate Elementary School science demonstrations and science fair, Bethesda, MD (1987-1994). Fallsmead Elementary School science fair, Rockville, MD (1996-1997) Science Mentor: Thomas Jefferson High School for Science and Technology: Adam Reese (Life Sciences and Biotechnology, 1996-1997) Shirlene Tsay (1998-1999) (First place winner in annual science fair; regional candidate in national competition) University Service: Molecular Pathobiology Program, Director, Department of Pathology, 1990-95. Medical Center Task Force (Improving Clinical Research at Georgetown), Chairman: Dr. Paul Katz, Dept. Medicine, Nov. 5 1991. Pathology Research Seminar Series, Organizer, 1990-1992. Annual Leadership Retreat, The Sciences and Biomedical Research at Georgetown, September 2021, 1991,Chantilly, VA. Lombardi Cancer Center Research Seminar Organizer (Ob/Gyn), 1992-95. Graduate Advisory Committee, 1993Pathology Grand Rounds Seminar Series Committee, 1994Pathology Advisory Committee, 1994-1997 Clinical Pathology Faculty Recruitment Committee, 1994. Committee for Restructuring Medical Student Pathology Course, 1994. Search Committee for Microbiology/Immunology Chairman, 1995, Chair: Dr. Marc Lippman. Executive Committee, Dept. Pathology, 1997. M.D./Ph.D. Program, Admissions and Mentorship Committee, Chairman, 1992-1997 Steering Committee, Biomedical Sciences Program, 1995-1998. Admissions and Curriculum Committee, Tumor Biology Program, 1993-1998. Medical Center Task Force (The future of research and graduate education at Georgetown) Chairman: Dr. Alan Faden, Jan. 1994 Pathology Graduate Program Committee, 1990-1997 Graduate Program Director’s Committee, 1993-1997 Steering and Admissions Committee, Molecular and Cellular Biology Graduate Program, 1993-1995 Antisense Consortium, Georgetown University Medical School, 1990-1994. Molecular Diagnostics Committee, Georgetown University Medical School, 1990-1993. Medical Center Task Force (Integration of the Biology Dept. into the M.D./Ph.D. program), March 1994, Chairman: Dr. Richard Schlegel Search Committee for the Chairman of Microbiology/Infectious Disease, Dr. Marc Lippman, Chair, February 1995-1997 Committee on Faculty, 1992 Investigative Committee (Massaro/Schlegel), Dr. John Griffith, Oct 1995. Task Force on Increased Research and Education Funding, Co-chair of Education subcommittee, February, 1997- May, 1997. Search committee for the Executive Vice President and Dean of the Medical School, Jan 2002-Dec 2003 Executive Committee of the Lombardi Comprehensive Cancer Center, 2005-2007. Search Committee for the Director of the Lombardi Comprehensive Cancer Center, 2007. Co-director, Growth regulation of cancer program, Lombardi Comprehensive Cancer Center, 2008. Associate directors committee, Lombardi Comprehensive Cancer Center, 2008. Graduate Student Thesis Committees for: Vivien Bubb (Pathology) Lex Cowsert (Microbiology) Robert Olson (Pathology) Xian Wen Jin (Pathology) Laufey Amundadottir (Anatomy and Cell Biology) Thorkell Andresson (Pathology) Xiangqun Zheng (Biology) Tony Wlazlo (Pathology) Alex Adduci (Pathology) Gary Disbrow (Tumor Biology Program) Tim Veldman (Tumor Biology Program) Vanessa Olcese (Tumor Biology Program) Kate Kierpiec (Microbiology and Immunology) Clare Thibodeaux (Tumor Biology Program) Aleksandra Dakic Sawali Sudarshan Rachel Condjella Jon Miller Teaching Activities: Courses: 175-513 Human tumor viruses and cancer. Course director and lecturer, Spring Semester (1992, 1994, 1996). 175-521 Neoplasia: A Survey. Lecturer, Fall Semester (1990). 175-501 Medical Pathology. Lecturer, neoplasia 4 neoplasia lectures (1990-present). 175-902 Tutorial Pathology. Lecturer (1991-present). Molecular Aspects of Neoplastic Hematopathology, Lecturer (1993-1997). 175-999 Thesis Research. 4225-506 Molecular and Experimental Pathology. Instructor (1992-2000.) Advisor, M.D./Ph.D. students: David Badawi Alex Adduci Vanessa Olcese Jon Miller Advisor, Ph.D. Graduate Program Vivien Bubb Thorkell Andresson Mark Mense Yan Chen Alexander Adduci (recipient of Howard Hughes Pre-doctoral fellowship) Mark Loveland Gary Disbrow Tim Veldman Vanessa Olcese Kate Kierpiec Clare Thibodeau Aleksandra Dakic Sawali Sudarshan Rachel Condjella Advisor, Biology Undergraduate thesis research Greg Lavecchia (senior research thesis) Brian Roehmholdt (senior research thesis) Kelly Goodman (Howard Hughes undergraduate research fellowship) Jess Hebert (Howard Hughes undergraduate research fellowship) Community outreach and media: Lombardi Comprehensive Cancer Center Straight Talk lectures to public in Maryland, Virginia and DC: 2006, 2007, 2008, 2009. John Carroll Society, “HPV vaccines: current and future”, Ft. Lauderdale, FL, April 2007. Radio: National Public Radio, Michel Martin host, Roughcuts, “Men and HPV”, www.npr.org/roughcuts, March 26, 2007. Newspaper: Washingtonpost.com; The HPV Vaccine; http://www3.georgetown.edu/gumc/update/17718.html or http://www.washingtonpost.com/wp-dyn/content/discussion/2007/01/16/DI2007011600929.html Radio: National Public Radio, Michel Martin host, Tell Me More, “HPV….and a few lighter notes”, May 10, 2007 U.S. Congressional briefing sponsored by the Congressional Prevention Caucus, Women in Government Relations and The American College of Obstetricians and Gynecologists: Rayburn building, “Everything you need to know about HPV”, May 22, 2007. U.S. Congressional briefing on Gynecologic Cancers and Disorders sponsored by the Women’s Caucus Taskforce on Health, the Congressional Cancer Caucus, the Caucus on Women’s Health, and including Fran Drescher of Cancer Schmancer. Cannon House Office Building, “The HPV vaccines”. September 25, 2007. Sarah Lawrence College lecture sponsored by the Lawrence Health Services and Georgetown University, Bronxville and Rye, New York. “Cervical Cancer Vaccines”. September 24, 2007. Georgetown University Blue and Gray Society, Sarasota, Florida, HPV vaccination, Nov 8-11, 2007. Georgetown University John Carroll Society, Madrid, Spain. Global initiatives for the prevention of HPV disease. March 6-8, 2008. American Health Journalism Conference, Arlington, VA. New developments in HPV vaccines. March 28, 2008. Junior League of Washington, Washington, DC. Current and future HPV vaccines. May 2008. Georgetown University, Witness to History, 2008. http://alumni.georgetown.edu/witnesstohistory Television: PBS, “To the contrary”. Hosted by Bonnie Erbe and including Dr. Bernadine Healy as panelist. HPV vaccines for boys. Taped Monday March 9, 2009. Aired April 5, 2009. www.pbs.org/ttc/podcasts.html Television: MMC TV16, "Today's Health " hosted by Michelle Katz. August 16, 2010. John Carroll Weekend, San Francisco, Georgetown University Alumni Association, Biotechnology: Past and Present, April 29, 2011 Georgetown University Alumni Association, Emerging Frontiers in Biotechnology, Medicine, and Regulatory Science: Panel and Networking Event, Bethesda, MD, June 28, 2011. Georgetown University Board of Regents, Georgetown and the HPV vaccine. Georgetown University Conference Center, Feb 3, 2012. John Carroll Weekend, Chicago, Georgetown University Alumni Association, Your Immortal Self. April 27-29, 2012. Georgetown Alumni Association, Class of 1962, Global Health Initiatives at Georgetown. June 2, 2012. NPR Radio: WAMU. Metroconnection. Georgetown Doc Works Toward Personalized Cancer Care. October 12, 2012. http://wamu.org/programs/metro_connection/12/10/12/georgetown_doc_works_toward_personalized _cancer_care Georgetown University Hospital Board of Directors, Washington DC, Conditionally reprogrammed cells: a new biology, a new Center, and a new biotechnology company, June 2013. Georgetown University Board of Regents, Washington DC, A new cell biology, a new Center, and a new biotechnology company, October 2013. MedStar Board of Directors, Eastern shore, MD, Clinical applications of HPV and cell reprogramming research, October 2013. Doctors Speak Out, Georgetown Conference Center, This One’s for You: The Future of Precision Medicine. December 3, 2013. Research at Georgetown University Medical Center: https://www.youtube.com/watch?v=rrvjENPDqDk&feature=youtu.be. August 14, 2014. Center for Cell Reprogramming: Redefining Personalized Medicine. Georgetown Medicine. Fall 2014. Research Grants (Active): “Emerging papillomaviruses in immunosuppressed dogs” Principal Investigator: Richard Schlegel Funding Agency: NIH Type: R01RR032315-01 Period: September 1, 2011-July 31, 2015 Annual direct costs: $390,000 Effort: 20% The major goal of this project is to isolate new papillomaviruses that are responsible for cutaneous tumors that arise in immunosuppressed dogs. In addition, once identified, appropriate vaccines will be generated for use in canine experiments that involve immunosuppression. This study will provide an animal model for the equivalent human disease. "Conditionally reprogrammed cells as a novel tool for biobanking" PI's: Richard Schlegel and Anton Wellstein Funding agency: NIH Type: R33 Duration: August 2013-July 2016 Annual direct costs: $300,000 Effort: 25% "Validating Conditionally Reprogrammed Cells to Advance Personalized Medicine in Prostate Cancer" PI: Chris Albanese Co-Investigator: Richard Schlegel Funding agency: Department of Defense RX 4424 808; PC 121 086 (proposal ID) Annual Direct Costs: Duration of grant: August 2013-July 2018 % Effort: 10% "Culturing Viably Captured Circulating Tumor Cells Using Conditional Reprogramming" PI: Richard Cote Co-investigator: Richard Schlegel Funding agency: NIH Annual direct costs: $175,000 Duration: 9/13-8/15 % Effort: 10% “Non-canonical functions of HTERT in cell immortalization by HPV” PI: Xuefeng Liu Co-investigator: Richard Schlegel Time commitments: 2.5% Supporting Agency: NIH R21 AI107510 Performance Period: 07/01/13-6/30/15 Level of Funding: $275,000 Research Grants (Completed): "Biology of the papillomavirus E5 oncoprotein family" Principal Investigator: Richard Schlegel, M.D., Ph.D. Agency: NIH, National Cancer Institute Type: R01 CA53371 Period: April 2006-February 2011 Annual direct costs: $280,000 Effort: 30% The major goal is to evaluate the biological properties of the human papillomavirus E5 oncoprotein and several other related viral proteins which appear to target the cellular V-ATPase. "HPV E6 protein regulation of the hTERT promoter" Principal investigator: Richard Schlegel M.D., Ph.D. Agency: NIH, National Cancer Institute Type: R01 CA106400 Period: September 2004-June 2010 Annual direct costs: $200,000 Effort: 20% This grant explores the mechanism by which the E6 oncoprotein of HPV induces the cellular telomerase gene, hTERT. Studies focus on the molecular interactions of E6 and Myc and on their physical binding to the hTERT promoter. "A next generation human papillomavirus vaccine" Principal Investigators: Dr. Robert Garcea, M.D. and Dr. Richard Schlegel, M.D., Ph.D. Agency: NIH, National Cancer Institute Type: RAPID award Period: October 1, 2001-September, 2003 (for laboratory studies), September 2003- (for clinical trials) Total direct costs (laboratory phase): inactive Effort: 0% (Future funding is via NIH contracts for GMP production of vaccine and the initiation and completion of Phase 1 human trials. Estimates range from 1-3 million) The major goal of this grant is to demonstrate the efficacy of an injectable COPV GST-L1 fusion protein which is produced in bacteria and capable of preventing canine mucosal infections. This animal model will serve as a model for transition into clinical trials with an analogous human vaccine for HPV-16 GST-L1. This grant will examine the in vitro and in vivo activity of artemisinin for treating papillomavirusinfected and –transformed cells. A canine model will be used to verify the in vivo activity of this compound. "Treatment of persistent papillomavirus infections with a therapeutic vaccine" Principal Investigator: Robert Garcea Co-investigators: Richard Schlegel, Luisa Villa, Lutz Gissman Agency: Gates Foundation (Grand Challenges in Global Health) Period: Jan 2006-Dec 2011 Total costs: 3.5 M for four research centers Annual direct costs for Schlegel laboratory: $90,000 (for three of five years) Effort: 10% This is a multi-institutional proposal to investigate the use of GST-L1-E7 fusion proteins (vaccine) to treat existing papillomavirus infections. "TICIPS: HIV/AIDS, secondary infections and immune modulation" Principal Investigator: William Folk Co-investigator: Richard Schlegel Agency: NCCAM, NIH Period Jan 2006-Dec 2009 Annual direct costs: $89,000 Effort: 5% This is a multinational grant focused on the evaluation of indigenous plants for the treatment of AIDS, TB and cervical cancer in Africa. "Cellular and Molecular Mechanisms of Gastrointestinal Cancers." Principal investigator: Lopa Mishra, M.D. Agency: NIH Type: P01 CA130821-01 Period: Dec. 2008-Nov 2013 Total costs: 9.1 M Project 3 Co-Principal Investigators: Richard Schlegel and Bibhuti Mishra Annual direct costs: $150,000 Effort: 10% "Topical treatments for genital papillomavirus infections" Principal investigator: Richard Schlegel Agency: NIAID, NIH Type: RFA-AI-06-005 Period: Sept. 2006-Aug. 2008 Annual direct costs: $150,000 Effort: 10% This is a two-tier grant to develop a canine genital papillomavirus infection model that will allow the screening of topical therapeutics. "Use of tobacco for medicinal purposes" USDA research grant (Production of a papillomavirus vaccine in tobacco plants) Funding period: July 1, 2002-June 30, 2003 Total direct costs: $463,000 (10% effort) “Papillomavirus E5 oncoprotein and cell transformation” Principal Investigator, Richard Schlegel M.D., Ph.D. NIH research grant 2R01CA53371 Funding period: 2001-2006 Total direct costs: $1,474,546 (60% effort). “Canine Oral Papillomavirus: Model for a Vaccine” Principal investigator, Richard Schlegel M.D., Ph.D. NIH grant 2R01CA57994 Funding period: July 1, 1997-June 30, 2001 Total direct costs: $847, 334 (20% effort) “A DNA Conformation Dependent Regulator of Gene Expression” Principal investigator, Richard Schlegel M.D., Ph.D. NIH grant R01CA75079 Funding period: July 1, 1999-June 30, 2000 Total direct costs: $236,021 “Adenovirus vectors for a papillomavirus vaccine” Co-Investigator, Richard Schlegel M.D., Ph.D. American Cancer Society grant Principal investigator, Dr. Gary Ketner, Johns Hopkins University Funding period: July 1996-June 1999 Total direct costs: $270,000 (5% effort) “Human papillomavirus E5 proteins” Sponsor, Physician scientist award 1K11CA01626-01 Melissa Conrad-Stoppler Funding period: April. 1992-March. 1997. Total direct costs: $463,061 (10% effort for sponsor for first year). “Antigenic determinants of the papillomavirus L1 capsid protein.” Co-Principal Investigator, Richard Schlegel M.D., Ph.D. NIH grant R01-CA47624 Principal investigator: Bennett Jenson Funding period: July 1989-June 1992. Total funding: $256,000. “Papillomavirus E5 oncoprotein and cell transformation” Principal Investigator, Richard Schlegel M.D., Ph.D. NIH research grant 1R01CA53371 funding period: Jan 1991-Dec 2001, total direct costs: $1,054,773, (60% effort). Academic/Industrial Collaborations: Active: Propagenix, Inc. Identification of Bioactive Factors Responsible for Conditional Reprogrammed Cell Cultures Funding period: June 2014 - June 2015 Total funding: $196,490 Completed: Pharmagenics, Inc. Antisense therapeutics for the human papillomaviruses funding period: 1991-1993. Total costs: $50,000 / year. MedImmune, Inc. Development of a vaccine for human papillomaviruses funding period: 1995-1996 Direct funding: $72,000 Millennium Pharmaceuticals Molecular diagnostics for cervical cancer Funding period: 2000-2002 Total funding $50,000 Patents and Licenses: Use of artemisinin and related compounds for treating diseases induced by human papillomavirus, European Patent No. 10183629.4-2123 / 2305243 Papillomavirus Vaccines, European Patent No. 07013259.2-1212 Vaccine) (First Generation HPV Papillomavirus Vaccines, U.S. Patent Application Pending, Application No. 08/216,506, filed June 25, 1992. Licensed to MedImmune, Inc. and GlaxoSmithKline. (First Generation HPV Vaccine) Stable (fixed) forms of viral capsid proteins, and viral capsid protein fusions, preferably papillomavirus L1 proteins, and uses thereof, U.S. Patent No. 7,279,306. (Second Generation HPV Vaccine) Human papillomavirus genes and their use in gene therapy, U.S. Patent No. 5,376,542 filed April 27, 1992. Minimal immunodominant epitopes of bovine papillomavirus type 1 L1 protein and the use thereof for the molecular tagging of cloned proteins. Licensed to BAbCo. Protecting against canine oral papillomavirus, U.S. Patent 5,874,089. (Animal papillomavirus vaccines.) Diagnosis and treatment of cervical cancer. (provisional patent) HPV E6 protein interacts physically and functionally with the cellular telomerase complex. Inventors: Richard Schlegel, Xuefeng Liu U.S. Patent Application Pending, Application No. 61/253,492, filed on October 20, 2009 Methods for extending lifespan and/or immortalizing epithelial cells. Inventors: Richard Schlegel and Xuefeng Liu U.S. Provisional Patent Application, application number: 61/413,291, filed on Nov. 12, 2010 U.S. Provisional Patent Application, application number: 61/474,901, filed on April 13, 2011 Immortalization of epithelial cells and methods of use. PCT Patent Application, application number: PCT/US11/60378, filed on Nov. 11, 2011 Inventors: Richard Schlegel and Xuefeng Liu Use of Artemisinin for Treating Tumors Induced by Oncogenic Viruses and for Treating Viral Infections U.S. Patent Application, Application Number: 13/777,244, filed on February 26, 2013 Compositions and Methods for Immortalization of Epithelial Cells. Inventors: Richard Schlegel, Frank Suprynowicz and Dan Paul Hartmann U.S. Provisional Patent Application, application number: 62/011,573, filed on June 13, 2014 Publications: Original papers 1. Schlegel, R. and Slade, H. D.: Determination of the rate of transformation from growth curves of transformed streptococci. J. Bacteriol. 111: 199-202, 1972. 2. Schlegel, R. and Slade, H. D.: Bacteriocin production by transformable group H streptococci. J. Bacteriol. 112: 824-829, 1972. 3. Schlegel, R. and Slade, H. D.: Properties of a streptococcus sanguis (Group H) bacteriocin and its separation from the competence factor of transformation. J. Bacteriol. 115: 655661, 1973. 4. Schlegel, R. and Slade, H. D.: Alteration of macromolecular synthesis and membrane permeability by a Streptococcus sanguis bacteriocin. J. Gen. Microbiol. 81:275-277,1974. 5. Duncan, J. L. and Schlegel, R.: Effect of Streptolysin 0 on erythrocyte membranes, liposomes, and lipid dispersions: a protein-cholesterol interaction. J. Cell Biol. 67:160-173, 1975. 6. Schlegel, R., Schaffhausen, B., Fluck, M., Silver, J., and Benjamin, T.: The hr-t function of polyoma virus. In Proceedinqs of the EMBO-INSERM Workshop, Early Proteins of Oncogenic DNA Viruses, Grignon, France, 69: 39-48, 1977. 7. Schlegel, R. and Benjamin, T.: Cellular alterations dependent upon the polyoma virus hr-t function: separation of mitogenic from transforming capacities. Cell 14: 587-599, 1978. (Cover article) 8. Schlegel, R., Banks-Schlegel, S., and Pinkus, G.: Immunohistochemical localization of keratin in normal human tissues. Lab. Invest. 42: 91-96, 1980. 9. Schlegel, R. Banks-Schlegel, S., McLead, J., and Pinkus, G.: Immunoperoxidase localization of keratin in human neoplasms. Am. J. Pathol. 101:41-48, 1980. 10. Willingham, M., Rutherford, A., Gallo, R., Wehland, J., Dickson, R., Schlegel, R., and Pastan, I.: Receptor-mediated endocytosis in cultured fibroblasts: cryptic coated pits and the formation of receptosomes. J. Histochem. Cytochem. 29: 1003-1013, 1981. 11. Banks-Schlegel, S., Schlegel, R., and Pinkus, G.: Keratin protein domains within the human epidermis. Exp. Cell Res. 136: 465-469, 1981. 12. Schlegel, R., Willilngham, M., and Pastan, I.: Monensin blocks endocytosis of vesicular stomatitis virus. Biochem. Biophys. Res. Commun. 102: 992-998, 1981. 13. Schlegel, R., Dickson, R., Willingham, M., and Pastan, I.: Amantadine and dansylcadaverine inhibit vesicular stomatitis virus uptake and receptor-mediated endocytosis of alpha2-macroglobulin. Proc. Nat. Acad. Sci. USA 79:2291-2295, 1982. 14. Dickson, R., Schlegel, R., Willingham, M., and Pastan, I.: Reversible and irreversible inhibitors of clustering of alpha2-macroglobulin in clathrin-coated pits on the surface of fibroblasts. Exp. Cell Res. 140: 215-225,1982. 15. Dickson, R., Schlegel, R., Willingham, M., and Pastan, I.: Binding and internalization of alpha2-macroglobulin by cultured fibroblasts. Effects of monovalent ionophores. Exp. Cell Res. 142: 127-140, 1982. 16. Schlegel, R., Willingham, M. C., and Pastan, I.: Saturable binding sites for vesicular stomatitis virus on the surface of Vero cells. J. Virol. 43:871-875, 1982. 17. Dickson, R., Schlegel, R., Willingham, M., and Pastan, I.: Involvement of Na+ and HC03- in receptor-mediated endocytosis of alpha2-macroglobulin, epidermal growth factor, and vesicular stomatitis virus. J. Cell. Physiol.113: 353-358,1982. 18. Schlegel, R., Tralka, T. S., Willingham, M. C., and Pastan, I.: Inhibition of VSV binding and infectivity by phosphatidylserine: Is phosphatidylserine a VSV-binding site? Cell 32: 639646, 1983. (Cover article) 19. Schlegel, R. and Wade, M.: Neutralized vesicular stomatitis virus binds to host cells by a different “receptor.” Biochem. Biophys. Res. Commun. 114:774-778, 1983 20. Schlegel, R., Banks-Schlegel, S., Vimadalal, S., and Said, J.: Keratins in carcinoid tumors. Lab. Invest. 49: 511-512, 1983. 21. Eidelman, 0., Schlegel, R., Tralka, T., and Blumenthal, R.: pH-dependent fusion induced by vesicular stomatitis virus glycoprotein reconstituted into phospholipid vesicles. J. Biol. Chem. 259: 4622-4628, 1984. 22. Schlegel, R. and Wade, M.: A synthetic peptide corresponding to the NH2-terminus of vesicular stomatitis virus glycoprotein is a pH-dependent hemolysin. J. Biol. Chem. 259: 4691-4694, 1984. 23. Schlegel, R. and Wade, M.: Biologically active peptides of the vesicular stomatitis virus glycoprotein. J. Virol. 53: 319-323, 1985. 24. Yee, C., Krishnan-Hewlett, I., Baker, C. C., Schlegel, R., and Howley, P.M.:Presence and expression of human papillomavirus sequences in human cervical carcinoma cell lines. Am. J. Path. 119: 361-366, 1985. 25. Schlegel, R.: Papillomaviruses and cervical cancer. Innov. in Oncol. 13-14, November, 1985. 26. Schlegel, R., Glass, M., Rabson, M. S., and Yang, Y.-C.: The E5 transforming gene of bovine papillomavirus encodes a small, hydrophobic polypeptide. Science 233:464-467, 1986. 27. Burkhardt, A., DiMaio, D., and Schlegel, R.: Genetic and biochemical definition of the bovine papillomavirus E5 transforming protein. EMBO J. 6: 2381-2385, 1987. 28. Zhang, Y.-L., Lewis, A., Wade-Glass, M. J., and Schlegel, R.: Levels of bovine papillomavirus RNA and protein expression correlate with variations in the tumorigenic phenotype of hamster cells. J. Virol. 61: 2924-2928,1987. 29. Schlegel, R.: Papillomaviruses. Arch. Dermatol. 123: 537, 1987. 30. McBride, A. A., Schlegel, R. and Howley, P. M.: The carboxy-terminal domain shared bythe bovine papillomavirus E2 transactivation and repressor proteins contains a specific DNA binding activity. EMBO J. 7:533-539, 1988. 31. Bubb, V., McCance, D.J., and Schlegel, R.: DNA sequence of the HPV-16 E5 ORF and the structural conservation of its encoded protein. Virology 163:243-246, 1988. 32. Howley, P. M., and Schlegel, R.: The human papillomaviruses: An Overview. Am. J. of Med. 85:155-158, 1988. 33. Schlegel, R., Phelps, W., Zhang, Y. L., and Barbosa, M.: Quantitative keratinocyte assay detects two biological activities of human papillomavirus DNA and identifies viral types associated with cervical carcinoma. EMBO J. 7: 3181-3187, 1988. 34. Horwitz, B. H., Burkhardt, A., Schlegel, R., and DiMaio, D.: 44 amino acid E5 transforming protein of bovine papillomavirus requires a hydrophobic core and specific carboxyl-terminal amino acids. Mol. Cell. Biol. 8: 4071-4078,1988. 35. Burkhardt, A., Willingham, M., Gay, C., and Schlegel, R.: The papillomavirus E5 oncoprotein is oriented asymmetrically in Golgi and plasma membranes. Virology 170: 334339, 1989. 36. Munger, K., Phelps, W.C., Bubb, V., Howley, P.M., and Schlegel, R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J. Virol. 63:4417-4421, 1989. 37. Barbosa, M., and Schlegel, R.: The E6 and E7 genes of HPV-18 are sufficient for inducing two-stage in-vitro transformation of human keratinocytes. Oncogene 4:1529-1532, 1989. 38. Goldstein, D., and Schlegel, R.: The E5 oncoprotein of bovine papillomavirus binds to a 16 kilodalton cellular protein. EMBO J. 9: 137-146, 1990. 39. Pietenpol, J.A., Stein, R.W., Moran, E., Yaciuk, P., Schlegel, R., Lyons, R.M., Pittelkow, M.R., Munger, K., Howley, P.M., and Moses, H.L.: TGF-beta1 inhibition of c-myc transcription in keratinocytes is abrogated by DNA tumor virus transforming proteins with intact binding domains for the retinoblastoma gene product. Cell 61: 777-785, 1990. 40. Villa, L., and Schlegel, R.: Differences in transformation activity between HPV-18 and HPV16 map to the viral LCR E6-E7 region. Virology 181: 374-377, 1991. 41. Romanczuk, H., Villa, L.V., Schlegel, R., and Howley, P.: The differential immortalization activities of human papillomavirus types 16 and 18 are determined by viral transcriptional regulatory regions upstream of the E6 and E7 genes. J. Virol. 65: 2739-2744, 1991. 42. Goldstein, D., Finbow, M.E., Andresson, T., McLean, P., Smith, K., Bubb, V., and Schlegel, R.: The bovine papillomavirus E5 oncoprotein binds to the 16 kilodalton component of vacuolar H+-ATPases. Nature 352: 347-349, 1991. 43. Bernstein, E.F., Glass, J.M., DeGraff, W.G., Schlegel, R., Black, C., Fisher, J.M., Cook, S.N., Glatstein, E., Russo, A., and Mitchell, J.B.: In-vitro photodynamic treatment of normal and human papillomavirus-transfected keratinocytes with photofrin II and red light. Arch. Dermatol. 127: 683-687, 1991. 44. Goldstein, D.J., Kulke, R., DiMaio, D., and Schlegel, R.: A glutamine residue in the membrane-associating domain of the bovine papillomavirus type 1 E5 oncoprotein mediates its binding to a transmembrane component of the vacuolar H+-ATPase. J. Virol. 66: 405413, 1992. 45. Toyama, R., Goldstein, D.J., Schlegel, R., and Dhar, R.: A genomic sequence of the Schizosaccharomyces pombe 16 kD vacuolar H+-ATPase. Yeast 7: 989-991, 1992. 46. Villa, L.L., Vieira, K., Pei, X.-F., and Schlegel, R.: Differential effect of tumor necrosis factor on the proliferation of primary human keratinocytes and cell lines containing human papillomavirus types 16 and 18. Mol. Carcinogenesis 6: 5-9, 1992. 47. Ghim, S., Jenson, A. B., and Schlegel, R.: HPV-1 L1 protein expressed in cos cells displays conformational epitopes found on intact virions. Virology 190:548-552, 1992 48. Goldstein, D., Toyama, R., Dhar, R., and Schlegel, R.: The BPV-1 E5 oncoprotein expressed in Schizosaccharomyces pombe exhibits normal biochemical properties and binds to a conserved 16 kDa component of the vacuolar proton-ATPase. Virology 190:889893, 1992. 49. Goldstein, D., Andresson, T., Sparkowski, J., and Schlegel, R.: The BPV-1 E5 protein, the 16 kD membrane pore-forming protein, and the PDGF receptor exist in a complex that is dependent on hydrophobic transmembrane interactions. EMBO J. 11:4851-4859, 1992. 50. Yankaskas, J., Haizlip, J., Conrad, M., Koval, D., Lazarowski, E., Paradiso, A., Rinehart, C., Sarkadi, B., Schlegel, R., and Boucher, R.: Papillomavirus immortalized tracheal epithelial cells retain a well-differentiated phenotype. Am. J. Physiology 264 (Cell Physiology 33): C1219-1230, 1993 51. Cohen, B., Goldstein, D., Rutledge, L., Vass, W., Lowy, D., Schlegel, R., and Schiller, J. Transformation-specific interaction of the bovine papillomavirus E5 oncoprotein with the platelet derived growth factor transmembrane domain and the epidermal growth factor cytoplasmic domain. J. Virol. 67: 5303-5311, 1993. 52. Pei, X.-F., Greenhalgh, D., and Schlegel, R.: Co-transfection of HPV-18 and v-fos DNA induces tumorigenicity of primary human keratinocytes. Virology 196: 855-860, 1993. 53. Conrad, M., Bubb, V. J., and Schlegel, R.: The human papillomavirus type 6 and 16 E5 proteins are membrane-associated proteins which associate with the 16-kilodalton poreforming protein. J. Virol. 67: 6170-6178, 1993. 54. Franchini, G., Mulloy, J., Koralnik, I., LoMonico, A., Sparkowski, J., Andresson, T., Goldstein, D., and Schlegel, R.: The human T-cell leukemia/lymphotropic virus type I p12I protein cooperates with the E5 oncoprotein of bovine papillomavirus in cell transformation and binds the 16-kilodalton subunit of the vacuolar H+ ATPase. J. Virol. 67: 7701-7704, 1993. 55. Steinman, K., Pei, X.-F., Stoppler, H., Schlegel, R., and Schlegel, R.: Elevated expression and activity of mitotic regulatory proteins in human papillomavirus-immortalized keratinocytes. Oncogene 9: 387-394, 1994. 56. Conrad, M., Goldstein, D., Andresson, T., and Schlegel, R.: The E5 protein of HPV-6, but not HPV-16, associates with cellular growth factor receptors. Virology 200: 796-800, 1994 57. Goldstein, D.J., Li, W., Wang, L.-M., Heidaran, M., Shinn, R., Aaronson, S., Schlegel, R., and Pierce, J.H.: The BPV-1 E5 transforming protein specifically binds and activates the PDGF receptor but not other related tyrosine kinase-containing receptors to induce cellular transformation. J. Virol.68: 4432-4441, 1994. 58. Pei, X.-F., Qin, N.G., Meck, J.M., and Schlegel, R.: Keratinocytes immortalized by human papillomavirus-18 exhibit alterations dependent upon host genetic background and complexity of viral genes transfected. Pathobiology 62:43-52, 1994. 59. Sparkowski, J., Anders, J., and Schlegel, R.: Mutation of the bovine papillomavirus E5 oncoprotein at amino acid 17 generates both high- and low- transforming variants. J. Virol. 68:6120-6123, 1994. 60. Greenhalgh, D.A., Wang, X.J., Rothnagel, J.A., Eckhardt, J.N., Quintanilla, M.I., Barber J.L., Bundman, J.S., Longley, M.A., Schlegel, R., and Roop, D.: Transgenic mice expressing targeted HPV-18 E6 and E7 oncogenes in the epidermis develop verrucous lesions and spontaneous, RASHA activated papillomas. Cell Growth Differ 5: 667-675, 1994. 61. Hines, J.F., Ghim, S.J., Christensen, N.D., Kreider, J.W., Barnes, W.A., Schlegel, R., and Jenson, A.B.: Role of conformational epitopes expressed by human papillomavirus major capsid proteins in the serologic detection of infection and prophylactic vaccination. Gynecol. Oncol. 55: 13-20, 1994. 62. Christensen, N.D., Kirnbauer, R., Schiller, J.T., Ghim, S.-J., Schlegel, R., Jenson, A.B., and Kreider, J.W. Human papillomavirus types 6 and 11 have antigenically distinct stronglyimmunogenic conformationally-dependent neutralizing epitopes. Virology 205: 329-335, 1994. 63. Hines, J.F., Ghim, S.-J., Christensen, N.D., Kreider, J.W., Barnes, J.W., Schlegel, R., and Jenson, A.B.: The expressed proteins of HPV-1, HPV-6 and HPV-11 display type-specific epitopes with native conformation and reactivity with neutralizing and non-neutralizing antibodies. Pathobiology 62: 165-171, 1994. 64. Bell, J., Sundberg, J.P., Ghim, S.J., Newsome, J., Jenson, A.B., and Schlegel, R.: A formalin-inactivated vaccine protects against mucosal papillomavirus infection: a canine model. Pathobiology 62: 194-198, 1994. 65. Andresson, T., Sparkowski, J., Goldstein, D.J., and Schlegel, R.: Vacuolar H+ -ATPase mutants transform cells and define a binding site for the papillomavirus E5 oncoprotein. J. Biol. Chem. 270: 6830-6837, 1995. 66. Sparkowski, J., Anders, J., and Schlegel, R.: E5 oncoprotein retained in the endoplasmic reticulum / cis Golgi still induces PDGF-receptor autophosphorylation but does not transform cells. EMBO J.14: 3055-3063, 1995. 67. Staebler, A., Pierce, J.H., Brazinski, S., Heidaran, M.A., Li, W., Schlegel, R., and Goldstein, D.J.: Mutational analysis of the beta type platelet-derived growth factor receptor defines the site of interaction with the bovine papillomavirus type 1 E5 transforming protein. J. Virol. 69: 6507-6517, 1995. 68. Suzich, J.A., Ghim, S.-J., Palmer-Hill, F.J., White, W.I., Tamura, J.K., Bell, J.A., Newsome, J.A., Jenson, A.B., and Schlegel, R.: Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci USA 92: 11553-11557, 1995. 69. Sparkowski, J., Mense, M., Anders, J., and Schlegel, R.: E5 oncoprotein transmembrane mutants dissociate fibroblast transforming activity from 16K binding and PDGF-R binding and phosphorylation. J. Virol. 70: 2420-2430, 1996. 70. Stöppler, H., Conrad-Stöppler, M., Adduci, A., Koval, D., and Schlegel, R.: The serine protease inhibitors, TLCK and TPCK, react specifically with the Rb-binding core of HPV-18 E7 protein and abolish its Rb-binding capability. Virology 217: 542-553, 1996. 71. Sherman, L., and Schlegel, R.: Serum / calcium-induced differentiation of human keratinocytes is inhibited by the E6 oncoprotein of human papillomavirus type 16. J. Virol. 70: 3269-3279, 1996. 72. Conrad-Stöppler, M., Schlegel, R., and McCance, D.: The E5 gene of HPV-16 enhances keratinocyte immortalization by full-length DNA. Virology 223: 251-254, 1996. 73. Conrad-Stöppler,M., Ching, K., Stöppler, H., Clancy, K., Schlegel, R., and Icenogle, J.: Natural variants of the HPV-16 E6 protein differ in immortalizing activity and ability to degrade p53. J. Virol. 70: 6987-6993, 1996. 74. Stöppler, H., Koval, D., and Schlegel, R.: The serine protease inhibitors TLCK and TPCK inhibit the in vitro immortalization of primary human keratinocytes by HPV-18 DNA. Oncogene 13: 1545-1548, 1996. 75. Stöppler, H., Hartman, D., Sherman, L., and Schlegel, R.: The human papillomavirus type E6 and E7 oncoproteins dissociate cellular telomerase activity from the maintenance of telomere length. J. Biol. Chem. 272: 13332-13337, 1997. 76. Grendys, E.C. Jr., Barnes, W.A., Weitzel, J., Sparkowski, J., and Schlegel, R.: Identification of H, K, and N-ras point mutations in stage IB cervical carcinoma. Gynecol. Oncol. 65: 343347, 1997. 77. Sherman, L., Jackman, A., Itzhaki, H., Conrad Stoppler, M, Koval, D., and Schlegel, R.: Inhibition of serum and calcium induced differentiation of human keratinocytes by HPV-16 E6 oncoprotein: role of p53 inactivation. Virology 237: 296-306, 1997. 78. Magal, S., Jackman, A., Pei, X.F., Schlegel, R., and Sherman, L. Induction of apoptosis in human keratinoctyes containing mutated p53 alleles and its inhibition by both the E6 and E7 oncoproteins. Int. J. Cancer 75: 96-104, 1998. 79. Li, J.J., Rhim, J.S., Schlegel, R., Vousden, K.H., and N.H. Colburn.: Expression of dominant negative jun inhibits elevated AP-1 and NF-kB transactivation and suppresses anchorageindependent growth of HPV immortalized human keratinocytes. Oncogene 16:2711-2721, 1998. 80. X-F Pei, Sherman, L., Y-H Sun, and Schlegel, R.: HPV-16 E7 protein bypasses keratinocyte growth inhibition by serum and calcium. Carcinogenesis 19:1481-1486, 1998. 81. Chen, Y., Ghim, S.-J., Jenson, A.B., and Schlegel, R.: Mutant L1 capsid proteins of the canine oral papillomavirus form virus-like particles but do not express native conformational epitopes. J. Gen. Virol. 79:2137-2146, 1998. 82. Stöppler, H., Conrad Stöppler, M., Johnson, E., Simbulan-Rosenthal, C.M., Smulson, M.E., Iyer, S., Rosenthal, D., and Schlegel, R.: The E7 protein of human papillomavirus type 16 sensitizes primary human keratinocytes to apoptosis. Oncogene 17:1207-1214, 1998. 83. Saito, T., Schlegel, R., Andresson, T., Yuge, L. Yamamoto, M., and Yamasaki, H. Induction of cell transformation by mutated 16K vacuolar H+ ATPase (ductin) is accompanied by down-regulation of gap junctional intercellular communication and translocation of connexin 43 in NIH3T3 cells. Oncogene 17:1673-1680, 1998. 84. Sundberg, J.P., Schlegel, R., and Jenson, A.B. Mucosotropic papillomavirus infections. Lab. Anim. Sci 48: 240-242, 1998. 85. Alfandari J, Schnitman Magal S, Jackman A, Schlegel R, Gonen P, Sherman L. HPV16 E6 oncoprotein inhibits apoptosis induced during serum-calcium differentiation of foreskin human keratinocytes. Virology. 1999 May 10;257(2):383-96. 86. AdduciA.J., and Schlegel, R.: The transmembrane domain of the E5 oncoprotein contains functionally discrete helical faces. J. Biol. Chem. 274: 10249-10258, 1999. 87. Schapiro, F., Sparkowski, J., Adduci, A., Suprynowicz, F., Schlegel, R., and Grinstein, S. Golgi alkalinization by the papillomavirus E5 oncoprotein. J. Cell Biol. 148: 305-315, 2000. 88. Suprynowitcz, F., Sparkowski, J., Baege, A., and Schlegel, R. E5 oncoprotein mutants activate phosphoinositide 3-kinase independently of platelet-derived growth factor receptor activation. J. Biol. Chem. 275: 5111-5119, 2000. 89. Ghim, S., Newsome, J., Sundberg, J.P., Schlegel, R., and Jenson, A.B. Spontaneously regressing oral papillomas induce systemic antibodies that neutralize canine oral papillomavirus. Exp. Mol. Pathol. 68: 147-151, 2000. 90. Yuan H, Estes PA, Chen Y, Newsome J, Olcese VA, Garcea RL, Schlegel R. Immunization with a pentameric L1 fusion protein protects against papillomavirus infection. J Virol. 75(17):7848-53, 2001. 91. Veldman T, Horikawa I, Barrett JC, Schlegel R. Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein. J Virol. 75(9):4467-72, 2001. 92. Carr EA, Theon AP, Madewell BR, Hitchcock ME, Schlegel R, Schiller JT. Expression of a transforming gene (E5) of bovine papillomavirus in sarcoids obtained from horses. Am J Vet Res. 62(8):1212-7, 2001 93. Suprynowicz, F., Baege, A., Sunitha, I., and Schlegel, R. c-src activation by the E5 oncoprotein enables transformation independently of PDGF receptor activation. Oncogene 21(11):1695-706, 2002. 94. Sherman L, Itzhaki H, Jackman A, Chen JJ, Koval D, Schlegel R. Inhibition of Serum- and Calcium-Induced Terminal Differentiation of Human Keratinocytes by HPV 16 E6: Study of the association with p53 degradation, inhibition of p53 transactivation, and binding to E6BP. Virology 292(2):309-20, 2002. 95. Baege, A., Berger, A., Schlegel, R., Veldman, T., and Schlegel, R. Cervical epithelial cells transduced with the papillomavirus E6/E7 oncogenes maintain stable levels of oncoprotein expression but exhibit progressive, major increases in hTERT gene expression and telomerase activity. Am. J. Pathol., 160: 1251-1257, 2002. 96. Simbulan-Rosenthal, C.M., Velena, A., Veldman, T., Schlegel, R., and Rosenthal, D.S. HPV E6/E7 immortalization sensitizes human keratinocytes to UVB by altering the pathway from caspase-8 to caspase-9-dependent apoptosis. J. Biol. Chem. 277:24709-24716, 2002. 97. Yuan, H., Veldman, T., Rundell, K, and Schlegel, R. SV-40 small tumor antigen activates AKT and telomerase and induces anchorage-independent growth of human epithelial cells. J. Virol. 76(21): 10685-91, 2002. 98. Berger, A.J., Baege, A., Guillemette, T., Deeds, T., Meyer, R., Disbrow, G., Schlegel, R., and Schlegel, R. Insulin-like growth factor binding protein 3 expression increases during immortalization of cervical keratinocytes by human papillomavirus type 16 E6 and E7 proteins. Am. J. Pathol. 161(2):603-610, 2002. 99. Rosenthal, D.S., Velena, A., Chou, F.P., Schlegel, R., Ray, R., Benton, B., Anderson, D., Smith, W.J., Simbulan-Rosenthal, C.M. Expression of dominant-negative FADD blocks human keratinocyte apoptosis and vesication induced by sulfur mustard. J. Biol. Chem. Mar 7;278(10):8531-40, 2003. 100. Wlazlo, A.P., Sparkowski, J.J., Jenson, A.B., and Schlegel, R. Expression of the bovine papillomavirus type 1 E5B gene reveals a protein-protein interaction of the E5A and E5B gene products. Virology 307: 395-405, 2003. 101. Veldman T, Liu X, Yuan H, Schlegel R. Human papillomavirus E6 and Myc proteins associate in vivo and bind to and cooperatively activate the telomerase reverse transcriptase promoter. Proc Natl Acad Sci U S A. Jul 8;100(14):8211-6, 2003. 102. Disbrow GL, Sunitha I, Baker CC, Hanover J, Schlegel R. Codon optimization of the HPV16 E5 gene enhances protein expression. Virology. Jun 20;311(1):105-14, 2003. 103. Baege, A, Disbrow, G, and Schlegel, R. IGFBP-3, a marker of cellular senescence, is overexpressed in HPV-immortalized cervical cells and enhances IGF-1-induced mitogenesis. J. Virol Jun;78(11):5720-7, 2004. 104. Olcese, VA, Chen, Y. Schlegel, R., Yuan. H. Characterization of HPV16 L1 loop domains in the formation of a type-specific, conformational epitope. BMC Microbiol. 2004 Jul 19. 105. Disbrow, GL, Hanover, J, and Schlegel, R. Endoplasmic reticulum-localized HPV-16 E5 protein alters endosomal pH but not trans-Golgi pH. J. Virology :5839-5846, 2005 106. Liu, X, Yuan, H, Fu, B, Disbrow, GL, Apolinario, T, Tomaic,V, Kelley, ML, Baker, CC, Huibregtse, J and Schlegel, R: The E6-AP ubiquitin ligase is required for transactivation of the hTERT promoter by the human papillomavirus E6 oncoprotein. J. Biol. Chem. 280(11):10807-10816, 2005. 107. Suprynowicz, FA, Disbrow, GL, Simic, V, and Schlegel, R. Are transforming properties of the bovine papillomavirus E5 protein shared by E5 from high-risk human papillomavirus type 16? Virology 332:102-113, 2005. 108. Magal, SS, Jackman A, Ish-Shalom, S, Botzer, LE, Gonen, P, Schlegel, R, and Sherman, L. Down regulation of Bax mRNA expression and protein stability by the E6 protein of human papillomavirus 16. J. Gen. Virol. 86:611-621, 2005. 109. Berg, M, DiFatta, J, Hoiczyk, E, Schlegel, R, and Ketner, G. Viable adenovirus vaccine prototypes: high-level production of a papillomavirus capsid antigen from the major late transcriptional unit. Proc. Natl. Acad. Sci USA 102:4590-4595, 2005. 110. Uren, A, Fallen, S, Yuan, H, Usubutun, A, Kucukali, T, Schlegel, R, Toretsky, JA. Activation of the canonical wnt pathway during genital keratinocyte transformation: a model for cervical cancer progression. Cancer Res. 65:6199-6206, 2005. 111. Williams, SM, Disbrow, GL, Schlegel, R, Lee, D, Threadgill, DW, and Lambert, PF. Requirement of epidermal growth factor receptor for hyperplasia induced by E5, a high risk HPV oncogene. Cancer Res. 65:6534-6542, 2005. 112. Disbrow, GL, Baege, AC, Kierpiec, KA, Centeno, JA, Hartmann, D-P, and Schlegel, R. Dihydroartemisinin is cytotoxic for papillomavirus-expressing epithelial cells in vitro and in vivo. Cancer Res. 65(23): 10854-61, 2005. 113. Zhang, Y, Fan, S, Meng, Q, Ma, Y, Katiyar, P, Schlegel, R, Rosen, E. BRCA1 interaction with human papillomavirus oncoproteins. J. Biol. Chem. 280(39):33165-77, 2005. 114. Saha, T, Vardhini, D, Tang, Y, Daturi, V, Jogunoori, W, Volpe, EA, Haines, D, Sidawy, A, Zhou, X, Gallicano, I, Schlegel, R, Mishra, B, Mishra, L. RING finger-dependent ubiquitination by PRAJA is dependent on TGF-beta and potentially defines the functional status of the tumor suppressor ELF. Oncogene 25(5):693-705, 2006. 115. Goldschmidt MH, Kennedy JS, Kennedy DR, Yuan H, Holt DE, Casal ML, Traas AM, Mauldin EA, Moore PF, Henthorn PS, Hartnett BJ, Weinberg KI, Schlegel R, Felsburg PJ. Severe papillomavirus infection progressing to metastatic squamous cell carcinoma in bone marrow-transplanted X-linked SCID dogs. J Virol. Jul;80(13):6621-8, 2006. 116. Mani O, Boweden ET, Lahusen T, Lorick KL, Weissman AM, Schlegel R, Wellstein A, and Riegel, AT. E6AP mediates regulated proteasomal degradation of the nuclear receptor coactivator amplified in breast cancer 1 in immortalized cells. Cancer Res. 66(17):8680-6, 2006. 117. Yuan H, Ghim S, Newsome J, Apolinario T, Olcese V, Martin M, Delius H, Felsburg P, Jenson AB, and Schlegel R. An epidermotropic canine papillomavirus with malignant potential contains an E5 gene and establishes a unique genus. Virology 359(1): 28-36, 2007. 118. Liu, X, Disbrow, GL, Yuan, H, Tomaic, V, and Schlegel, R. Myc and HPV-16 E7 proteins cooperate to immortalize human keratinocytes. J. Virol. 2007 Nov;81(22):12689-95. Epub 2007 Sep 5. 119. Suprynowicz, FA, Disbrow, GL, Krawczyk, E, Simic, V, Lantzky, K, and Schlegel, R. The HPV-16 E5 oncoprotein upregulates lipid raft components caveolin-1 and ganglioside GM1 at the plasma membrane. Oncogene. 2008 Feb 14;27(8):1071-8. Epub 2007 Aug 20. 120. Liu, X, Roberts, J, Dakic, A, Zhang, Y, Quintero, J, Baker, C, and Schlegel, R. HPV E7 contributes to the telomerase activity of immortalized and tumorigenic cells and augments E6-induced hTERT promoter function. Virology. 2008 June 5; 375(2):611-623. Epub Mar 24. (Ranked in the top 10 recent virology articles: http://top25.sciencedirect.com/subject/immunology-and microbiology/14/journal/virology/00426822/archive/18/ 121. Krawczyk, E, Hanover, J, Schlegel, R, and Suprynowicz, FA. Karyopherin beta 3: a new cellular target for the HPV-16 E5 protein. Biochem. Biophys. Res. Comm. 2008 Jul 11; 371:684-688. Epub 2008 May 1. 122. Krawczyk, E, Suprynowicz, FA, Liu, X, Dai, Y, Hartmann, DP, Hanover, J, and Schlegel, R. Koilocytosis: a cooperative interaction between the HPV E5 and E6 oncoproteins. Am. J. Path. 2008 Sep; 173(3):682-688. Epub 2008 Aug 7. (cover article) 123. Liu, X, Dakic, A, chen, R, Disbrow, GL, Dai, Y, Zhang, Y, Simic, V, and Schlegel, R. Cellrestricted immortalization by HPV correlates with telomerase activation and Myc engagement of the hTERT promoter. J. Virol. 2008 Dec 82(23):11568-76. [Epub ahead of print, Sep 25]. (selected as “Spotlight” publication by editors) 124. Thibodeaux, CA, Liu, X, Disbrow, GL, Zhang, Y, Rone, JD, Haddad, BR, and Schlegel, R. A tumor-derived Myc gene is sufficient to immortalize and transform primary mammary epithelial cells. Breast Cancer Res. Treat. 2009 Jul 116(2):281-94. [Epub ahead of print, Jul 20, 2008]. 125. Condjella R, Liu X, Suprynowicz F, Yuan H, Sudarshan S, Dai Y, Schlegel R. The canine papillomavirus E5 protein signals from the endoplasmic reticulum. J. Virol. 2009 Dec 83(24):12833-41. [Epub Oct 7, 2009] 126. Krawczyk E, Suprynowicz FA, Sudarshan SR, Schlegel R. Membrane orientation of the human papillomavirus type 16 E5 oncoprotein. J Virol. 2010 Feb 84(4):1696-1703. [Epub Dec 2, 2009] 127. Liu X, Dakic A, Zhang Y, Dai Y, Chen R, Schlegel R. HPV E6 protein interacts physically and functionally with the cellular telomerase complex. Proc Natl Acad Sci U S A. 2009 Nov 3;106(44):18780-5. Epub 2009 Oct 20. 128. Chapman S, Liu X, Meyers C, Schlegel R, McBride AA. Human keratinocytes are efficiently immortalized by a Rho kinase inhibitor. J Clin Invest. 2010 Jul 1;120(7):2619-26.PMID: 20516646 129. Peran I, Riegel A, Dai Y, Schlegel R, Liu X. Is HPV-18 present in human breast cancer cell lines? Br J Cancer. 2010 May 11;102(10):1549-50; author reply 1551-2. Epub 2010 Apr 20. PMID: 20407441 130. Suprynowicz FA, Krawczyk E, Hebert JD, Sudarshan SR, Simic V, Kamonjoh CM, Schlegel R. The HPV-16 E5 Oncoprotein Inhibits EGF Trafficking Independently of Endosome Acidification. J Virol. 2010 Oct;84(20):10619-29. Epub 2010 Aug 4. 131. Sudarshan SR, Schlegel R, Liu X The HPV-16 E5 protein represses expression of stress pathway genes XBP-1 and COX-2 in genital keratinocytes.. Biochem Biophys Res Commun. 2010 Sep 3;399(4):617-22. Epub 2010 Aug 3. 132. Wang J, Zhou D, Prabhu A, Schlegel R, Yuan H. The canine papillomavirus and gamma HPV E7 proteins use an alternative domain to bind and destabilize the retinoblastoma protein. PLoS Pathog. 2010 Sep 2;6(9):e1001089. PMID:20824099. 133. Krawczyk E, Suprynowicz FA, Hebert JD, Kamonjoh CM, and Schlegel R. The HPV-16 E5 Oncoprotein Translocates Calpactin I to the Perinuclear Region. J Virol. 2011 Nov; 85(21):10968-75. Epub 2011 Aug 17. PMID:21849434. (Selected as “Spotlight” publication by editors) 134. Jagu S, Malandro N, Kwak K, Yuan H, Schlegel R, Palmer KE, Huh WK, Campo MS, Roden RB. A multimeric L2 vaccine for prevention of animal papillomavirus infections. Virology 2011 Nov 10; 420(1):43-50. Epub Sept 13 2011. PMID:21920572 135. Ringer L, Sirajuddin P, Heckler M, Ghosh A, Suprynowicz F, Yenugonda V, Brown M, Toretsky J, Uren A, Lee Y, McDonald T, Rodriguez O, Glazer R, Schlegel R, and Albanese C. VMY-1-103 is a novel CDK inhibitor that disrupts chromosome organization and delays metaphase progression in medulloblastoma cells. Cancer Biol Ther. 2011 Nov1; 12(9)81826. Epub 2011 Nov 1. PMID:21885916. (Cover article) 136. Liu X, Ory V, Chapman S, Yuan H, Timofeeva OA, Albanese C, Haddad B, Rhim JS, Dritschilo A, Riegel A, McBride A, Schlegel R. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012 Feb; 180(2):599-607. Epub 2011 Dec 18. PMID:22189618. (Selected for Commentary) 137. Yuan H, Luff J, Zhou D, Wang J, Affolter V, Moore P, Schlegel R. 2012. Complete genome sequence of canine papillomavirus type 9. J Virol. 2012 May;86(10):5966. 138. Al-Otaiby M, Tassi E, Schmidt M, Chien C, Baker T, Salas A, Xu J, Furlong M, Schlegel R, Riegel A, and Wellstein A. Role of the nuclear receptor coactivator AIB1/SRC3 in angiogenesis and wound healing. Am J Pathol. 2012 Apr; 180(4): 1474-84. Epub 2012 Feb 14. 139. Bulut G, Fallen S, Beauchamp E, Drebing LE, Sun J, Berry DL, Kallakury B, Crum CP, Toretsky JA, Schlegel R, Üren A. Beta-catenin accelerates human papillomavirus mediated cervical carcinogenesis in transgenic mice. PLoS One. 2011; 6(11):e27243. Epub 2011 Nov 7. 140. Bonilla-Delgado J, Bulut G, Liu X, Cortés-Malagón EM, Schlegel R, Flores-Maldonado C, Contreras RG, Chung SH, Lambert PF, Uren A, Gariglio P. The E6 Oncoprotein from HPV16 Enhances the Canonical Wnt/β-catenin Pathway in Skin Epidermis in vivo. Mol Cancer Res. 2012 Feb; 10(2): 250-8. Epub 2011 Dec 7. 141. Low G, Attiga YS, Garg G, Schlegel R, Gallicano GI. Can male vaccination reduce the burden of human papillomavirus-related disease in the United States? Viral Immunology 2012 (in press). 142. Sahab Z, Sudarshan S, Liu X, Zhang Y, Kirilyuk A, Kamonjoh C, Simic V, Dai Y, Byers S, Doorbar J, Suprynowicz R, and Schlegel R. Quantitative measurement of HPV-16 E5 protein in cell lines by mass spectrometry. J Virol. 2012 Sep;86(17):9465-73. Epub 2012 Jun 27. PMID:22740411 143. Luff J, Moore P, Zhou D, Wang J, Usuda Y, Affolter V, Schlegel R, Yuan H. Complete genome sequence of canine papillomavirus type 10. J Virol. 2012 Oct;86(20):11407. PMID:22997424. 144. Yuan H, Meyers S, Wang J, Zhou D, Woo J, Kallakury B, Ju, A, Bazylewicz M, Carater, Y, Albanese C, Grant N, Shad A, Dritschilo A, Liu X, and Schlegel R. Use of reprogrammed cells to identify therapy for respiratory papillomatosis. N Engl J Med 2012 Sept 27; 367(13): 1220-7. 145. Sirajuddin P, Das S, Ringer L, Rodriguez OC, Sivakumar A, Lee YC, Uren A, Fricke ST, Rood B, Ozcan A, Wang SS, Karam S, Yenugonda V, Salinas P, Petricoin E 3rd, Pishvaian M, Lisanti MP, Wang Y, Schlegel R, Moasser B, Albanese C. Quantifying the CDK inhibitor VMY-1-103's activity and tissue levels in an in vivo tumor model by LC-MS/MS and by MRI. Cell Cycle. 2012 Sep 14;11(20). [Epub ahead of print] 146. Suprynowicz F, Upadhyay G, Krawczyk E, Kramer S, Hebert JD, Liu X, Yuan H, Chaitra Cheluvaraju C, Phillip W. Clapp PW, Boucher Jr. RC, Kamonjoh CM, Randell SH and Schlegel R. Conditionally reprogrammed cells represent a stem-like state of adult epithelial cells. Proc Natl Acad Sci USA 2012 Dec 4; 109(49): 20035-40. 147. Yuan H, Liu, X, and Schlegel R. Reprogrammed cells for respiratory papillomatosis (letter). N Engl J Med 2012 Dec 27; 367(26): 2553-2554. 148. Bove PF, Dang H, Cheluvaraju C, Jones LC, Liu X, O'Neal WK, Randell SH, Schlegel R, Boucher RC. Breaking the In-Vitro Alveolar Type II Cell Proliferation Barrier While Retaining Ion Transport Properties. Am J Respir Cell Mol Biol. 2013 Nov 5. [Epub ahead of print] PMID:24191670 [PubMed - as supplied by publisher] 149. Luff JA, Yuan H, Suter MM, Müller EJ, Schlegel R, Moore PF. Canine keratinocytes upregulate type I interferons and proinflammatory cytokines in response to poly(dA:dT) but not to canine papillomavirus. Vet Immunol Immunopathol. 2013 Jun 15;153(3-4):177-86. doi: 10.1016/j.vetimm.2013.02.001. Epub 2013 Mar 5. PMID:23557936 150. Miller J, Dakic A, Chen R, Palechor-Ceron N, Dai Y, Kallakury B, Schlegel R, Liu X. HPV16 E7 Protein and hTERT Proteins Defective for Telomere Maintenance Cooperate to Immortalize Human Keratinocytes. PLoS Pathog. 2013 Apr;9(4):e1003284. doi: 10.1371/journal.ppat.1003284. PMID:23592995. 151. Yuan H, Zhou D, Wang J, and Schlegel R. Divergent human papillomavirus associated with recurrent respiratory papillomatosis with lung involvement. Genome Announc. 2013 Jul 11; 1(4):e00474-13. doi: 10.1128/genomeA.00474-13. 152. Pelchor-Ceron, N., Supyronowicz, F., Upadahay, G., Dakic, A., Minas, T., Simic, V., Johnson, M., Albanese, C., Schlegel, R., Liu, X. Radiation Induces Diffusible Feeder Cell Factor(s) That Cooperate with ROCK Inhibitor to Conditionally Reprogram and Immortalize Epithelial Cells, Am J Pathol. 2013 Dec;183(6):1862-70. doi: 10.1016/j.ajpath.2013.08.009. Epub 2013 Oct 3. 153. Pollock CB, McDonough S, Wang VS, Lee H, Ringer L, Li X, Prandi C, Lee RJ, Feldman AS, Koltai H, Kapulnik Y, Rodriguez OC, Schlegel R, Albanese C. Yarden RI. Strigolactone Analogues Induce Apoptosis Through Activation of p38 and the Stress Response Pathway in Cancer Cell Lines and in Conditionally Reprogramed Primary Prostate Cancer Cells. Oncotarget. 2014 Apr 2; Epub ahead of print. PMID:24742967. 154. Zheng YL, Zhang F, Sun B, Du J, Sun C, Yuan J, Wang Y, Tao L, Kota K, Liu X, Schlegel R, Yang Q. Telomerase Enzymatic Component hTERT Shortens Long Telomeres in Human Cells, Cell Cycle. 2014 Apr 10;13(11). Epub ahead of print. PMID: 24721976. 155. Zhou D, Luff J, Usada Y, Affolter V, Moore P, Schlegel R, Boucher RC. Complete genome sequence of canine papillomavirus type 11. Genome Announc. 2014 May 29;2(3).PMID:24874662 156. Luff JA, Yuan H, Kennedy D, Schlegel R, Felsburg P, Moore PF. Keratinocyte antiviral response to Poly(dA:dT) stimulation and papillomavirus infection in a canine model of Xlinked severe combined immunodeficiency. PLoS One. 2014;9(7). PMID25025687 157. Saenz FR, Ory V, AlOtaiby M, Rosenfield S, Furlong M, Cavalli LR, Johnson MD, Liu X, Schlegel R, Wellstein A, Reigel AT. Conditionally repgrogrammed normal and transformed mouse mammary epithelial cells display a progenitor-cell like phenotype. PLoS One. 2014;9(5). PMID:24831228 Publications: Reviews and chapters. Schlegel, R.: Membrane-active peptides of the vesicular stomatitis virus glycoprotein. In LonbergHolm, K. and Crowell, R. L. (Eds.): Virus Attachment and Entry into Cells. Washington, D. C., ASM Publications, 66-73, 1986. Howley, P. M. and Schlegel, R.: Papillomavirus transformation. In N. P. Salzman and P.M. Howley (Eds.): Papovaviridae. 2. The Papillomaviruses, New York, Plenum Press, 1986, pp. 141-166. Schlegel, R.: Probing the function of viral fusion proteins with synthetic peptides. In A. Sowers (Ed.) Cell Fusion, New York, Plenum Press, 33-43,1987. Schlegel, R., and Wade-Glass, M.: The E5 transforming protein of bovine papillomavirus. In Steinberg, B., Brandsma, J., and Taichman, L. (Eds.): Cancer Cells, New York, Cold Spring Harbor Laboratory Press, 1987, pp. 87-91. Phelps, W.C., Munger, K., Yee, C.L., Schlegel, R., and Howley, P.M.: The genital human papillomaviruses: transcriptional regulation and transformation. In: Common mechanisms of transformation by small DNA tumor viruses. Ed. L.P. Villareal, ASM Publications, Washington, DC, pages 149-164, 1989. Munger, K., Phelps, W.C., Bubb, V., Howley, P.M., and Schlegel, R.: Keratinocyte transformation by the HPV-16 E6 and E7 genes progresses through two distinct morphological stages. In: Papillomaviruses, UCLA Symposium on Molecular and Cellular Biology, New Series, Volume 124, pages 223-230, Editors, P. Howley and T. Broker, Alan R. Lissk Inc., New York, NY, 1989. Schlegel, R.: Papillomaviruses and human cancer. In: Viral pathogenesis (ed. Fujinami, R.), Seminars in Virology 1: 297-306, 1990. Howley, P.M., Munger, K., Werness, B., Phelps, W.C., and Schlegel, R.: Molecular mechanisms of tranformation by the human papillomaviruses, Genetic basis for carcinogenesis: tumor suppressor genes and oncogenes, A.G. Knudson, Jr. et.al. (eds.),Japan Sci. Soc. Press, Tokyo/Taylor and Francis Ltd., London pp 199-206, 1990. Cossman, J., and Schlegel, R.: p53 in the diagnosis of human neoplasia. JNCI 83: 87-88, 1991. Finbow, M., Pitts, J., Goldstein, D., Schlegel, R., and Findlay, J.: The E5 oncoprotein target: a 16 kD channel-forming protein with diverse functions. Mol.Carcinogen. 4: 441-444, 1991 Conrad, M., Yankaskas, J., Boucher, R., and Schlegel, R.: Using the papillomavirus E6/E7 genes to generate well-differentiated epithelial cell lines. in: Neoplastic transformation in human cell systems in vitro: mechanisms of carcinogenesis, Humana Press, 1991. Ghim, S.-J., Schlegel, R., Hines, J., Christensen, N., Kreider, J., and Jenson, A.B. : Comparison of PV conformational epitopes expressed by L1 proteins in mammalian (cos) and insect (Sf9) cells; pages 147-153, in Immunology of Human Papillomaviruses (Ed. M.A. Stanley), Plenum Press, New York, 1994. Stoppler, H., Conrad-St”ppler, M., and Schlegel, R.: Transforming proteins of the papillomaviruses. Intervirology 37: 168-179, 1994. Hines, J.F., Ghim, S.-J., Schlegel, R., and Jenson, A.B.: Prospects for a vaccineagainst human papillomavirus. Obst. Gyn. 86: 860-866, 1995. Suprynowicz, F, Campo, S., and Schlegel, R. Biology of the papillomavirus E5 proteins, 2006. Goodrich S, Schlegel R, Guixang W, Belinson J. Use of Artemisinin and Its Derivatives to Treat HPV-Infected/Transformed Cells and Cervical Cancer: A Review, Future Oncol. 2014 647–654; 10(4). Presentations: 1. Third Conference on Differentiation Therapy. Speaker. Modulation of keratinocyte proliferation and differentiation: two dissociable activities of the human papillomaviruses. September 5-10, 1988, Villasimius, Sardinia. 2. Georgetown University, Department of Obstetrics and Gynecology, A quantitative in-vitro assay for the biological activity of human papillomaviruses. January 27, 1989. 3. University of Tennessee Medical School, Department of Pathology, Memphis, TN, Cellular transformation by human papillomaviruses. February 27-28, 1989. 4. University of Connecticut Health Center, Department of Microbiology, Keratinocyte transformation by papillomaviruses and the role of the E5 oncoprotein. February 22, 1989. 5. E.I. DuPont., Deleware, Keratinocyte transformation by the human papillomaviruses. April 19, 1989. 6. Armed Forces Institute of Pathology, Division of Molecular Diagnostics, Washington, DC, The role of human papillomaviruses in human neoplasia. May 16, 1989. 7. Triton Biosciences, Inc., Oakland, CA, Papillomavirus oncoproteins. June 5, 1989. 8. 1989 UCLA Symposium on “Papillomaviruses”. Symposium Speaker. Modulation of keratinocyte differentiation by human papillomaviruses. Taos, New Mexico. March 11-18, 1989. 9. Papillomavirus Workshop 1990. Oral presentation. The E5 oncoprotein of bovine papillomavirus bind to a 16 kd cellular protein. Heidelberg, Germany. May 12-17, 1990. 10. Harvard School of Public Health, Department of Toxicology, Boston, MA, Viruses and human neoplasia. February 5, 1990. 11. Georgetown University, Department of Microbiology and Immunology, HPV genes involved in human keratinocyte transformation. September 26, 1990. 12. Lombardi Cancer Center, Georgetown University Medical School, HPV and human neoplasia. November 30, 1990. 13. Georgetown University Medical School, Department of Obstetrics and Gynecology, Biological differences between HPV-16 and HPV-18, November 26, 1990. 14. International Symposium on Human Papillomavirus. Plenary session speaker. Different HPV genotypes have different transforming activities. Sao Paulo, Brazil. November 8-10, 1990. 15. International Papillomavirus Workshop. Plenary session speaker. HPV Transformation: Host-Cell Interactions. Seattle, Washington, July 20-26, 1991. 16. Gordon Conference on Epithelial Differentiation and Keratinization, Tilden School, New Hampshire, Speaker, Altered tumor suppressor gene expression in HPV-transformed keratinocytes, July 28-August 2, 1991. 17. Roswell Park Cancer Institute, Buffalo, NY, The transforming oncoproteins of the papillomaviruses, March 3-5, 1991. 18. Georgetown University Medical Center, A Workshop on Neoplastic Transformation in Human Cell Systems In-Vitro: Mechanisms of Carcinogenesis, The phenotype of human epithelial cells immortalized by the E6/E7 oncogenes of HPV-18, April 25-26, 1991. 19. American Medical Association and Georgetown University Medical School, Georgetown University Medical School, Polymerase Chain Reaction—A Diagnostic Tool for the 1990’s, moderator, October 11-12, 1991. 20. Fifth Annual HPV Conference, Chicago, IL, Speaker, The biology of the human keratinocyte, Oct. 25-28, 1992. 21. The Milton Hershey Medical Center, Pennsylvania State University, Hershey, PA., The papillomavirus E5 oncoprotein and signal transduction, Nov. 5-7, 1992. 22. Concepts in Molecular Biology, American Association of Pathology. Speaker. The Human Papillomaviruses, Bethesda, MD, October 29-November 1, 1992. 23. Gordon Reearch Conference, Drug Carriers in Biology and Medicine, Speaker, Molecular approaches to the prevention and treatment of HPV infections, New Hampshire, July 6-10, 1992. 24. Fifth International Conference on Human Papillomaviruses and Genital Carcinoma. Speaker. Basic Biology of the Human Cell, and Role of Human Tumor Suppressor Genes. Chicago, IL, October 26-28, 1992. 25. 11th International Papillomavirus Workshop, Session Chairman and Plenary session speaker, Edinburgh, Scotland, Sept. 5-12, 1992. 26. Northwestern University, Evanston, IL., Molecular interactions between the papillomavirus E5 oncoprotein, the platelet-derived growth factor receptor, and the proton pump. May 20, 1993. 27. Harvard University, Dept. Pathology, Brigham and Women’s Hospital, Boston, MA., The papillomavirus E5 oncoprotein: unique mechanisms for modulating the signal transduction system. October 2, 1993. 28. American Society of Investigative Pathology, Concepts in Molecular Biology, Bethesda, MD, Human Papillomaviruses, October 28-30, 1993. 29. Gordon Conference on Epithelial Differentiation and Keratinization, Tilden School, New Hampshire, Speaker, Papillomavirus proteins that modulate keratinocyte growth, August 16, 1993. 30. Annual AIDS Meeting, NCI, Laboratory of Tumor Cell Biology, Bethesda, MD, Speaker, The papillomavirus E5 oncoprotein and its role in signal transduction, August 22-28, 1993. 31. National Cancer Institute Cervical Cancer Workshop, Panelist, Molecular Biology of Cervical Cancer, September 23-24, 1993. 32. Mt. Sinai Medical Center, Dept. Microbiology, Molecular interactions of the papillomavirus E5 oncoprotein, March 22, 1994. 33. Albert Einstein College of Medicine, Long Island Jewish Medical Center, Dept. of Otolaryngology, Oncoproteins of the papillomaviruses, May 3, 1994. 34. Yale University, Department of Biophysics and Molecular Biology, Structural properties of the E5 oncoprotein, September, 1994. 35. International Papillomavirus Workshop, Amsterdam, Netherlands, A formalin-inactivated vaccine and recombinant L1 protein protect completely against mucosal papillomavirus infection: a canine model, October 8-12, 1994. 36. 14th International Papillomavirus Conference, Quebec City, Canada, The role of E5 in papillomavirus transformation, Guest speaker, July 23-28, 1995. 37. Symposium on “Cell cycle control and viral oncogenesis”, Heidelberg, Germany, Alterations in cyclins and cyclin-dependent kinases during cell immortalization by human papillomaviruses, Invited speaker, October 4-6, 1995. 38. Tufts University, Department of Biochemistry, Molecular mechanism of E5-induced activation of growth factor receptors, April, 1996. 39. International symposium on “Fifteen years’ progress and future perspectives of vacuolar ATPases”, Kanagawa, Japan, Invited speaker, November 2-5, 1996 40. Current Aspects of Vaccinology and Molecular Virology, Invited speaker, DIA Symposium, Dana Point, CA, April 9-11, 1997. 41. University of Maryland Medical School, Baltimore, MD, Receptor-dependent and independent mechanisms of cell transformation by the papillomavirus E5 oncoprotein. February 27, 1997. 42. USUHS Medical School, Bethesda, MD, Papillomavirus-mediated immortalization of human keratinocytes; The papillomavirus E5 oncoprotein: unique pathways to cell transformation. February 10, 1997. 43. 16th International Papillomavirus Conference, University of Siena, Italy, Sept. 5-12, 1997. Program Committee. 44. University of Maryland Medical School, Baltimore, MD, Dept. Pathology, Papillomaviruses: from genes to vaccines. October, 1998 45. Lombardi Cancer Center, Georgetown University Medical Center, Minisymposium on Cancer Vaccines, Papillomavirus vaccines: the importance of discontinuous epitopes, December 18, 1998. 46. Johns Hopkins Medical School, Grand Rounds in Pathology, Papillomaviruses: from genes to vaccines. April 16, 1998. 47. 17th International Papillomavirus Conference, Charleston, SC, Invited Speaker, January 5, 1999. 48. Indiana University, Department of Microbiology and Immunology, Cell transformation by the papillomavirus E5 oncoprotein. November, 1999. 49. Thomas Jefferson University, Department of Pathology, Telomerase induction by the papillomavirus E6 oncoprotein. Feb. 2002. 50. Harvard University, Brigham and Women’s Hospital, Pathology Grand Rounds, Molecular characterization of a second-generation papillomavirus vaccine. June 19, 2002. 51. 19th International Papillomavirus Conference, Florianopolos, Brazil, Session Chair, September, 2001 (cancelled). 52. Structural biology of small DNA tumor viruses, Siena, Italy, The papillomavirus E6 protein and cellular Myc protein co-modulate the hTERT promoter. April 21-25, 2003. 53. The Seventh International Conference on Malignancies in AIDS and Other Immunodeficiencies: Basic, Epidemiologic and Clinical Research. NIH, Natcher Conference Center. Plenary session speaker. The papillomavirus E6 oncoprotein, telomerase, and cell immortalization. April 28-29, 2003. 54. 20th International Papillomavirus Conference, Trieste, Italy. The papillomavirus E6 protein and cellular Myc protein co-modulate the hTERT promoter. July 15-20, 2003 55. New insights into Gastrointestinal Pathophysiology and Cancer, Washington, DC. Myc, telomerase and cell immortalization. Oct. 2-3, 2003. 56. Uniformed Services University of Health Sciences, Bethesda, MD, Department of Pathology, The papillomavirus E6 protein and cellular Myc protein co-modulate the hTERT promoter. March 14, 2003. 57. University of Iowa, Iowa. The papillomavirus E6 protein and cellular Myc protein co-modulate the hTERT promoter. April 7-9, 2003. 58. Cervical Cancer Workshop, National Centre for Biological Sciences, TIFR, Bangalore, India. Papillomaviruses and telomerase. 2004. Invited speaker (cancelled trip). 59. Medical College of Georgia Cancer Center, Augusta, GA, Oct. 18, 2004. Papillomaviruses, telomerase, and cell immortalization. Michigan State University, Michigan. E6, telomerase and cell immortalization. April 8, 2004. 60. Hershey Medical Center, Penn State University, Papillomaviruses and cervical cancer. July 15, 2004. 61. University of Maryland, Greenebaum Cancer Center, Papillomaviruses: from genes to vaccines, September 20, 2004. Cancer Nanotechnology Symposium, Lombardi Comprehensive Cancer Center, Washington, DC. Pentameric nanoparticles function as a highly effective vaccine platform. Feb. 22, 2005. 62. Center for Infectious Diseases, Georgetown University, moderator. Feb. 2005. 63. EMBO Workshop, Siena, Italy, Structural basis of papovavirus biology, moderator and speaker, The in vitro and in vivo activities of dihydroartemisinin as an anti-papillomavirus therapy. April 11-16, 2005. 64. 22nd International Papillomavirus Workshop, Vancouver, Canada, May 1-6, 2005 (2 oral presentations). 65. GlaxoSmithKline, Brussels, Belgium, March 14, 2005. Second generation papillomavirus vaccines. University of Massachusetts Medical School, Department of Pathology, Worcester, MA. May 19, 2005. 66. Novartis, Oncology Division, Cambridge, MA, Invited seminar speaker. Topic: Papillomaviruses, cell immortalization and control of the hTERT promoter. July 15, 2005. 67. American Cancer Society, Cervical Cancer Committee of the Maryland Comprehensive Cancer Control Plan, White Marsh, MD. Oral presentation on the state of prophylactic vaccines, May 9, 2005. 68. Center for Infectious Disease conference, Georgetown University, Keynote speaker, Global initiatives for treating and preventing cervical cancer. February 27, 2006. 69. Grand Challenges in Global Health, Gates Foundation, 2 nd Annual Meeting, Washington, DC, Oct. 4-6 2006. 70. GlaxoSmithKline Biologicals, Third Extramural R&D Symposium, Brussels, Belgium, Oct. 910 2006. 71. Deutscheskrebsforshungzentrum HPV Symposium, Heidelberg, Germany, Dec. 15, 2006. 72. American Academy of Allergy, Asthma, and Immunology (AAAAI) Annual meeting, Current and future HPV vaccines, Feb 23-27 2007, San Diego, CA 73. Intercampus Workshop Series. Exploring the HPV vaccine and the social, legal and public health debate. HPV vaccines: promise and problems. Georgetown University, March 12, 2007 74. Georgetown University Undergraduate Research Conference, Keynote Speaker, Secondgeneration HPV vaccines, March 22, 2007. 75. John Carroll society meeting, The future of HPV vaccines, Fort Lauderdale, Florida, April 1213, 2007. 76. Johns Hopkins University, Asthma and Allergy Center, Progress and Problems: the HPV vaccine, June 8, 2007 77. DNA tumor virus meeting, Trieste, Italy, The HPV E5 and E6 proteins cooperate to induce koilocytosis, July 2007 78. International Papillomavirus Meeting, Beijing, China, October 2007 (3 abstracts) 79. University of Virginia, Charlottesville, VA, Department of Pathology Grand Rounds, New membrane-localized activities of the HPV E5 oncoprotein. February 5, 2008. 80. Forsyth Institute, Boston, MA. New membrane-localized activities of the HPV E5 oncoprotein. February 14, 2008. 81. University of Missouri, Columbia, MO. Oncology Grand Rounds. New approaches to the prevention and treatment of HPV infections. March 3, 2008. 82. Georgetown University Medical Center, Ob/Gyn Grand Rounds, Current and future HPV vaccines, Feb 21, 2008. 83. Walter Reed Army Research Institute, Current and future HPV vaccines. Rockville MD. 84. Gates Foundation Meeting, April 7-8, 2008 85. Brigham and Women’s Hospital, Womens’ Annual Research Seminar. May 14, 2008. 86. University of California, Davis, HPV and cell immortalization: Bypassing the Hayflick limit, October 2010. 87. Sarah Stewart Memorial Lecture, Georgetown University Medical School, The new pathology, September 15, 2011. 88. International Papillomavirus meeting, Berlin Germany, Keynote Address: Cancer Stem Cells, September 2011. 89. National Cancer Institute, Laboratory of Molecular Biology, NIH, Bethesda, MD. CRC’s: the next-generation for cell culture. Feb 6, 2012. 90. Grand Rounds, Yale University Medical School, Department of Pathology, Conditionally reprogrammed cells (CRCs): cell culture for the next-generation Pathology department, February 16, 2012. 91. Next Generation Pathology (Molecular Medicine Triconference, Cambridge Healthtech Institute), San Francisco CA, Bringing biobanks to life: conditionally reprogrammed cells, February 19-20, 2012,. 92. National Cancer Institute, NIH, Bethesda, MD. Conditionally reprogrammed cells. March 20, 2012. 93. Stony Brook Medical Center, Marvin Kuschner Memorial Lecture for Advanced Cellular and Molecular Pathology, Pathology Grand Rounds, Stony Brook, Long Island. Conditionally reprogrammed cells: a new era for personalized medicine. March 29, 2012. 94. Case Western Reserve University, Lerner Research Institute, Translating HPV and cell reprogramming research into clinical applications. Keynote Speaker. April 3-4, 2012. 95. Wayne State University School of Medicine, Detroit, Michigan, Keynote Speaker: Translating HPV and cell reprogramming research into clinical applications. September 27, 2012. 96. Massachusetts General Hospital, Harvard University, Oncology Grand Rounds, Conditionally reprogrammed cells: the future of personalized medicine? April 9-10, 2013. 97. George Washington University Hospital, Hematology/Oncology Tumor Board, Conditionally reprogrammed cells for personalized medicine. March 15, 2013. 98. Dana Farber Cancer Center, Harvard University, Renal Cancer Spore Grant, Conditionally reprogrammed cells are adult stem-like cells. April 12, 2013. 99. Memorial Sloan Kettering Cancer Center, Prostate Think Tank, Conditional reprogramming for the study of prostate biology. April 25, 2013. 100. Johns Hopkins University Medical Center, Hematology/Oncology Grand Rounds, Conditionally reprogrammed cells and precision medicine. April 29, 2013. 101. NCI, NIH, Speaker at Workshop on organoids and related human cancer model systems. Conditionally reprogrammed cells for precision and regenerative medicine. August 28, 2013. 102. Edmund A. Walsh School of Foreign Service, Georgetown University, Maloy Distinguished Professor Lecture, Clinical translation of HPV and cell research. November 14, 2013. 103. Targeted Anticancer Therapies 2014 Conference, Plenary Session Speaker, Cell Reprogramming Applications to Targeted Therapies. March 7, 2014. 104. Stevens Institute. Keynote Speaker at the Stevens Symposium on Innovation in Healthcare Technology and Delivery, Cell Reprogramming. March 11, 2014. 105. American Medical Women’s Association, Guest Speaker, HPV, Cancer and Vaccines. March 15, 2014. 106. American Association Cancer Research Annual Meeting 2014. Conditional Reprogramming for the Real-Time Analysis of Tumor Biopsies. April 7, 2014. 107. University of Miami, Miller School of Medicine, Grand Rounds, Conditional Cell Reprogramming: Biology & Medical Applications. April 24, 2014. 108. Johns Hopkins University School of Medicine, Department of Otolaryngology-Head and Neck Surgery, invited speaker and activity co-director for Updates in Recurrent Respiratory Papilloma: Clinical and Research Perspectives, The HPV Vaccine: from Prophylaxis to Therapy. May 30, 2014. 109. ORIP, NIH, Seminar, Conditional Reprogramming Applications to Biology & Medicine. September 23, 2014. 110. Sanger Institute, Speaker at the Next Generations Cell Culture Model Workshop, Conditional Reprogramming Applications to Biology & Medicine. October 2, 2014. 111. University of Pennsylvania, Pathology and Laboratory Medicine, Guest Speaker for a Grand Rounds Series, Conditional Cell Reprogramming: The Next-Generation of Cell Culture for Biology and Medicine. October 13, 2014. 112. Mt. Sinai, Seminar Guest Speaker, Conditional Cell Reprogramming: The Next-Generation of Cell Culture for Biology and Medicine. November 25, 2014.