Western Blot detailed
advertisement

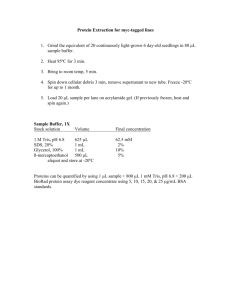
Melissa Landis 106739590 SDS-PAGE Assembling gel mold 1. 2. 3. 4. 5. 6. 7. Use 1 small and 1 large plate. Add spacers. Place in gel alignment position. Place gray gasket on top of red. Pop into holder. Mark 1 cm below teeth of comb. Pour EtOH to ensure tight seal. Pouring gel ***Polyacrylamide is a very potent neurotoxin ****wear gloves*** 1. Combine following reagents: Resolving gel (2) 30% acrylamide/bis ddH2O 1.5M Tris, pH8.8 20% SDS 10% APS Stacking gel (2) 30% acrylamide/bis ddH2O 0.5 M Tris, pH 6.8 20% SDS 10% APS 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 8% gel ml 5.3 9.4 5.0 0.1 0.2 20 10% gel ml 6.6 8.1 5.0 0.1 0.2 1.66 6.85 1.26 0.05 0.1 9.92 Add 30 l TEMED to resolving gel. Mix by pipetting. Pour to above drawn line. Add methanol to both corners. Allow to polymerize >30 min. Pour off methanol. Add 20 l TEMED to stacking gel. Pour, overfill, add combs. Polymerize 15 min. Remove combs. Wash with ddH20 to remove unpolymerized gel from wells. Place in tank. Fill tank with running buffer. Running Buffer 100ml 10X Running Buffer 5 ml 20% SDS Up to 1L with H20 10X Running Buffer 30.25g Trizma Base MW 121 144.25g Glycine MW 75.07 Up to 1L with H2O 2/16/16 Melissa Landis 106739590 2/16/16 Prepare samples & load samples Well number Sample name Concen (g/l) Volume loaded 1 2 3 4 5 6 7 8 9 10 Well number Sample name Amt (g) Volume loaded 1 2 3 4 5 6 7 8 9 10 1. 2. 3. 4. 5. 6. 7. 8. Boil 3 minutes (because SDS precipitates when frozen), flash spin. Thaw marker. Place in chamber. Load samples. Load 1X Laemmli sample buffer in empty lanes. Laod 20l BenchMark (Invitrogen) with 36 l 1X LSB on top. Fill container with run buffer. Run at 200V for 35-40 min until dye front reaches bottom. Melissa Landis 106739590 2/16/16 Transfer Transfer Buffer 100 ml 10X Running Buffer 100 ml methanol Up to 1L with H2O 1. Cut 5 X 9.5 cm PVDF membrane. **Wear gloves** Cut top right corner off. Label membrane with pencil. 2. Soak in MeOH 15min. 3. Gently separate gel from 1 plate, trim excess & stacking gel. 4. Soak PVDF, fiber filters, & gel in transfer buffer 10 min. 5. Layer on black side of holder: fiber, filter with gel, membrane, filter, fiber. Add transfer buffer & roll out any bubbles between each layer. 6. Place in holder black to black and clear to red. 7. Repeat for second gel. 8. Fill reservoir with transfer buffer. Place reservoir in ice bucket, start magnet spinning, add holder, ice pack. 9. Hook up red to red & black to black. 10. Run 100V 1H .35Amp max. 11. Mark membrane. Coomassie Stain Gel (Maniatis 18.55) 1. Add 5 volumes Coomassie Stain. Rock at RT min 4H. ***save stain**** 2. Destain in MeOH: H2O: Acetic acid (45:45:10) for 4-8 H rocking, changing solution 3-4X. The more destaining the more small amounts of protein can be detected.. 3. Photograph the gel. Fast Green Blot 1. Stain in fast green for 7 min, rocking. ***save fast green**** 2. Destain on rocking platform at RT: 2X- MeOH 2.5min, 5 min 3. Photograph the membrane. 4. Destain on rocking platform at RT: 3X H20 2X PBST Immunoblotting 1. Block with 50 ml 5% milk (in PBS-T) for 60 min., rocking at RT. Use 200l tip box. 2. Wash 3X with PBS-T 10 min, rocking at RT. 3. 30ml 1Ab (5% BSA,0.02%NaN3 in PBST) 4C o/n or 2-4H RT. **save 1Ab at 4C** 4. Wash 3X with PBS-T 10 min, rocking at RT. 5. 2Ab in 50ml 5% milk in PBS-T, 1 H at RT, rocking. 6. Start with 1:35,000 dilution If you do not get any signal after developing: repeat steps 4& 5 with less dilute antibody Proceed trying different dilutions until optimal: 1:20,000 1:10,000 1:5000 1:1000 Wash 3X with PBS-T 10 min, rocking at RT. ****If membrane becomes dry at any step rehydrate in MeOH**** Chemiluminescence & Developing 1. 2. Aliquot 1:1, ECL reagent 1&2. About 2ml total for 14cm x 17 cm membrane. Gather film, timer, scissors, and cassette. Melissa Landis 3. 4. 5. 6. 106739590 2/16/16 Combine reagents 1&2. Pipet across membrane 3-4X. Place in sheet protector, place in cassette, & press out bubbles. Cut film to appropriate size, fold upper right corner, & place in cassette. Leave 5 min initially. Develop. Stripping Membranes (Current protocols in Molecular Biology 10.8.14) 1. Wash blot in distilled water 5min. 2. Transfer to 0.2M NaOH and wash 5min. 3. Wash blot in distilled water 5 min. 4. Repeat chemiluminescence & developing to verify completing stripping. I actually extended the stripping time. You may have to adjust this for your specific blot. Melissa Landis 106739590 2/16/16 Blot name: Stripping Membranes (Current protocols in Molecular Biology 10.8.14) 1. 2. 3. 4. Wash blot in distilled water 5min. Transfer to 0.2M NaOH and wash 5min. Wash blot in distilled water 5 min. Repeat chemiluminescence & developing to verify completing stripping. I actually extended the stripping time. You may have to adjust this for your specific blot. Melissa Landis 106739590 2/16/16