Kinetics
advertisement

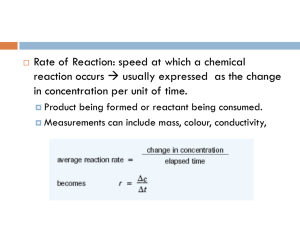
We have spent the past few months (!!) learning how to write chemical equations, balancing chemical equations, and using the stoichiometry to relate quantities of reactants and products in the chemical reaction. We have spent time studying the three main types of chemical reactions, acid-base, precipitation, and redox. And we have studied the heat, and entropy associated with chemical reactions. Another thing of importance to study is how fast does a reaction go, how much reactant is consumed or how much product is formed in a given amount of time, are there ways that we can speed up or slow down a chemical reaction, how do the atoms come together during the chemical reaction so that we can determine the path in a step-by-step manner known as the mechanism. The study of motion is called kinetics, from the Greek work kinesis, meaning “movement.” Kinetics deals with the speed of a reaction and its mechanism, or pathway that the molecules take to get from reactant to product. Chemical kinetics is the study of reaction rates, the changes in concentration per unit time. Every chemical reaction proceeds at a different rate. Some require a very long time to consume the reactants and are described as slow. The disintegration of an aluminum can by atmospheric oxidation or of a plastic bottle by the actions of sunlight can take years, decades, or even centuries. While other reactions occur in the blink of an eye. Acid base neutralization reactions occur as fast as we can mix the materials together. Hydrogen gas and fluorine gas at room temperature form hydrogen fluoride gas in an extremely rapid reaction. Hydrogen gas and oxygen gas forming water at room temperature happens to be extremely slow. Under conditions of high temperature however, the reaction occurs with much speed. Carbon monoxide and nitrogen monoxide, two harmful pollutants are produced in large quantities in automobile engines. These gases can react with one another and produce CO2 and N2 gas which are considerably more environmentally friendly than CO and NO. Unfortunately, this reaction is very slow, regardless of the temperature, but by using a catalyst (a species that helps the reaction along but itself is not consumed in the chemical reaction) in the form of the catalytic converter in your car, this reaction occurs at a much faster rate. Previously, we discussed catalysts with LeChatelier’s Principle. Catalysts are invaluable components when studying chemical kinetics or when performing chemical reactions. Knowing how fast a reaction occurs, or being able to control the speed of the reaction is very important in the real world. How fast can medicine act in the body? How long after you take that aspirin does your headache go away? How long after you take your allergy medicine will you stop sneezing? How long will the medicine stay in your system – how long does it take for the medicine to be metabolized? How long does it take for cement to harden? Nowadays that can be very important. Down in Corvallis, OR they actual pay people to sit and watch the cement dry to prevent delinquent youths from writing in the wet cement. The longer it takes the cement to dry, the more they have to pay people to literally sit there. In general, chemists have the ability to manipulate reaction speeds to some extent. Even a little manipulation of a chemical reaction can increase product yield, increase or decrease the speed of the reaction as 1 needed for a particular manufacturing process. The ability to manipulate the reaction can save the company millions of dollars thus increasing profit – a very important thing in society today. It was through kinetics and the study of mechanisms that the pathway leading to ozone depletion was discovered. The mechanism indicates that chlorine atoms from chlorofluorocarbons (CFCs) act as the catalysts in the depletion of the ozone layer. Confidence in the accuracy of the mechanism has led the nations of the world to take measures to reduce and hopefully eventually eliminate the use of CFCs. Without kinetics and reaction mechanism, the ozone layer may be even worse off than it is today. Rate is defined as a change per some unit time. The rate of motion of a moving car is defined in some number of miles per hour. A chemical reaction rate is expressed as a change in amount or concentration of some reactant or product per unit time. Time can be measured in seconds, minutes, hours, days, years, etc . . . depending on how long the reaction takes or even how long the scientist chooses to observe the reaction. Example units for rate: moles sec Molarity sec torr minute atm hour If one can predict reaction rates, chances are one can do things to control the rate. Certain variables are very important in controlling the rate of the reaction, such as the nature of the reactants, concentration of reactants, temperature, surface area and the presence of a catalyst. Under a given set of conditions (e.g. temperature and pressure) each reactant has its own characteristic rate. This is determined by the chemical nature of the reactants. At room temperature, hydrogen, the same reactant gas, behaves very differently chemically in the following chemical reactions: H2 (g) + F2 (g) → 2HF (g) [very fast] 3H2 (g) + N2 (g) → 2NH3 (g) [very slow] There is a common reactant in each chemical equation, H2, yet the reaction rates for the two reactions are nothing alike. This means that each reaction rate is unique. Generalizations should not be made (meaning that every time you see H2 gas does not mean that the reaction is always fast, or even that it is always slow). Limited predictions can be made however, they should be done so with caution: Ions in solution tend to react quickly: most ionic precipitates tend to form quickly. Think back to the chemical reactions demo when a tiny Pb(NO3)2 crystal and some KI crystals were placed on either side of a drop of water. When you moved the crystals into the drop of water, almost immediately you saw the formations of the yellow PbI2 solid. The same reaction occurred when pre-made solutions of Pb(NO3)2 and KI were mixed 2 together. The bright yellow precipitate appeared almost instantaneously. Acid-base neutralization reactions performed in lab occurred almost instantaneously. You all monitored the changes by using phenolphthalein indicator and the color change was also instantaneous. You did not have to wait 20 minutes or even 2 minutes to see if the reaction was complete. Ionic reactions are fast due to the high mobility of the dissolved ions (Brownian motion!!) and the electrostatic attraction that occurs between positively and negatively charged ions. Covalent molecules, especially large ones, tend to react slowly. Even in solution form, organic reactants or other covalent reactants can take hours or days to yield an appreciable amount of product. In organic next year, many of you will experience the joys of organic lab, as you wait hours upon hours for your product to be produced. Molecules with strong covalent bonds tend to react more slowly than those with weak bonds. O2 and N2 persist in the atmosphere and do not undergo chemical reactions because each has high bond dissociation energies. (think of their Lewis structures!) Other molecules, such as Cl2 have weak bond dissociation energies and tend to react much more quickly. Higher concentrations tend to react more quickly than species at low concentrations. Reactions can only occur when chemical species get near one another. At lower concentrations molecules have a hard time finding one another. If they cannot find one another, they cannot possibly be close enough to react. Imagine you want to find a person, ANY person on this campus and there are only 10 people total here one day. And they can be anywhere one campus. Good luck finding them! However, on a normal school day, how easy is it to find a person – once again ANY person with whom you can interact – pretty easy! Same with molecules that will be involved in a chemical reaction. reaction rate collisional frequency concentration The ability to “mix” affects reaction rate. The frequency of collision also depends on the physical state of the reactants. When the reactants are in the same phase (homogeneous reaction), such as the solid or liquid phase, the molecules are free to move around and to collide with one another. When the reactants are in different phases (heterogeneous reaction), then the reaction will occur at the interface between the two phases. The greater the surface area available between the reactants, the more contact there is and the faster the reaction can proceed. The more finely divided a solid or liquid reactant, the more surface area is available and the faster the reaction. Molecules must collide with enough energy in order to react. Remember that molecules in the gas phase move faster and with more average kinetic energy as the temperature is increased. More motion means more collisions. Many collisions result in a billiard ball like effect. The molecules collide and actually bounce off one another. No reaction takes place. Once enough energy is put into the system (usually in the form of increasing the temperature) the molecules have sufficient energy upon reaction colliding with one another to react. Raising the temperature increases the reaction rate by increasing the rate of collisions and more importantly, imparting enough energy to the molecules allowing them to react. reaction rate collisional energy temperature 3 The effects of temperature on reaction rate are intuitively known – even now – by you! What happens to milk if you leave it out? It spoils – probably pretty quickly as in a matter of days if you leave it on the counter. Why do we put it in the refrigerator? To SL-O-O-W down the rate of the chemical reaction known as spoiling. What if we want a good steak. How long would we have to wait if we took the meat out of the refrigerator for it to cook itself on the countertop? Right, it would spoil before it cooked itself!! So in order to cook the meat (also a chemical reaction) we put the meat on the grill – at a high temperature in order to brown the meat! A catalyst is a substance that increases the rate of the reaction without itself being consumed in the chemical reaction. Catalysts are widely used in industry to speed up reactions that would otherwise be too slow to be practical. They are also used to carry out processes at lower and more economical temperatures. A catalyst that works for one reaction may not work at all for another. Enzymes are catalysts for biochemical reactions, but each enzyme is used for a specific reaction in the biochemical pathway. There are literally thousands of enzymes present in living cells. In order to determine a reaction rate, you must measure the change in the amount of substance per unit time. You can measure the change in terms of the loss of the reactant or the gain in the amount of product per unit time. Concentration changes can be measured using a colorimeter during the reaction without disturbing the reaction process. Or, small samples of material can be taken and analyzed at fixed time intervals throughout the reaction process. Changes in ionic concentrations can be measured by electrical conductance. However, measuring these systems requires a reaction that proceeds at a slow enough pace in order to be able to observe the changes. A reaction that occurs within a matter of seconds proceeds too quickly and presents a challenge to scientists. Sometimes reactions can be literally frozen in time, by the addition of another reactant and by icing down the reaction. Studies involving proteins are often done in this manner. A protein will be mixed with another reactant, then an acid will be added to “quench” the system and the solution will be put on ice. While the reaction will continue to proceed, its rate is occurring on the minute to hour scale which allows the investigator a brief window of time in which the system can be examined as it. Permutations in protein structure, folding and unfolding, crosslinking, and polymerization are often studied in this manner. When observing chemical reactions, a key point to remember is that reactant concentrations decrease with time and product concentrations increase with time. Consider the following reaction: 2NO2 (g) → 2NO (g) + O2 (g) The rate of reaction can be examined by measuring the rate at which NO is formed, the rate at which O2 is formed, or by examining the rate at which NO2 disappears. Imagine if we measured the amount of NO gas in the reaction vessel. Sometime later, we take another measurement of the amount of NO present. The increase in concentration of NO is: 4 Δ[NO] = final concentration of NO – initial concentration of NO Δt = final time – initial time Rate of NO formation = Δ[NO] Δt The above expression of rate signifies the rate at which the NO concentration increases over some time interval. The reaction rate can also be expressed in terms of the formation of O2 gas. Rate of O2 formation = ΔO2 Δt Note that the rates of NO formation and O2 formation will be positive. The final concentrations will be larger than the initial concentration measurements and the final time will be larger than the initial time measurement. What about the disappearance of the reactant, NO2? If the concentrations of the products increase, then the concentration of the reactants will decrease. This means that mathematically, the final concentration of the reactant will be less than the initial concentration. This makes the change in the reactant concentration a negative number. The change in time will still be positive (final time will be larger than initial time). However, we cannot have a negative rate that really does not mean anything. A reaction rate is inherently positive. So when the reaction rates are written in terms of the reactants, we must take the absolute value, or the opposite of the sign so that all reaction rates are expressed as positive numbers. Rate of NO2 disappearance = - ΔNO2 Δt Thus, reaction rate = -rate of [NO2] disappearance Reaction rate = + [NO] appearance Reaction rate = + [O2] appearance The coefficients do mean something when examining the reaction rate. Again, this will come down to a point-of-view situation. Pick a point of view that makes sense to you – but keep in mind, the coefficients do tell us something about the relative rates of the formation of products and the disappearance of the reactants. 2NO2 (g) → 2NO (g) + O2 (g) rate of NO formation = 2x rate of O2 formation or rate of O2 formation = ½ the rate of NO formation 5 rate of NO formation = -rate of NO2 disappearance rate of O2 formation = - ½ rate of NO2 disappearance In other words, the ratios given above are NOT identical, but are related to the stoichiometric amounts of species such that Δ[NO] Δt = 2 ΔO2 Δt = _ Δ[NO2] Δt or 1Δ[NO] 2 Δt = ΔO2 Δt = _ 1Δ[NO2] 2 Δt The mathematical expression for the rate of a particular reaction and the numerical value of the rate depend on which substance you are using as the reference. In general, the rate for a particular reaction: aA + bB → dD + eE rate = -1 Δ[A] = -1 ΔB = +1[D] = +1[E] a b d e Concept Test: The stoichiometry for a given reaction can be used to calculate the rate for any reactant or product in the chemical reaction if another rate is known. 3NO2 (g) + H2O (l) → 2HNO3 (l) + NO (g) the nitrogen dioxide is consumed at a rate of 0.30 mol/Lsec. Express the reaction rate in terms of the concentration of nitric acid Remembering and using the stoichiometry of the balanced chemical reaction means that it does not matter which way you express the reaction rates (using fractions or not). Notice the two ways of expressing the reaction rates will numerically yield the same answers when used in 6 problems, but setting up the dimensional analysis problem the same way that we have for the past two terms means we will always get the correct answer. A typical kinetics experiment yields a series of concentration and time readings that must be converted into rates. In the nitrogen dioxide decomposition reaction, nitrogen dioxide is reddish-brown while the products, NO and O2 are both colorless. Thus, as the reaction proceeds, the color of the gas in the container slowly disappears. 2NO2 (g) → 2NO (g) + O2 (g) Examining the chemical reaction shows us that for every two molecules of NO 2 that disappear, 2 molecules of NO appear. Thus, there is a 1:1 molecular relationship between the chemical species. And for every two molecules of NO2 that disappear, one molecule of O2 appears. Let’s examine another chemical reaction: the reaction between ethene and ozone, which may contribute to ground level smog formation: C2H4 (g) + O3 (g) → C2H4O (g) + O2 (g) Examining only the reactant concentrations we can say that for every 1 molecule of ethene that we have, it will react with exactly 1 molecule of ozone. In other words, the concentrations of both reactants will decrease at the same rate. Supposed you start with a known amount of O3 in a closed reaction vessel kept at a constant temperature. You allow the reaction to proceed for 60 seconds, after which, the concentration of O3 has changed to a new value. The rate would therefore be equal to: Time (sec) 0.0 10.0 20.0 30.0 40.0 50.0 60.0 Rate = _ Δ[O3] Δt Concentration of O3 (mole/L) 3.20 x 10-5 2.42 x 10-5 1.95 x 10-5 1.63 x 10-5 1.40 x 10-5 1.23 x 10-5 1.10 x 10-5 Rate = _ Δ[O3] = _ 1.10 x 10-5 - 3.20 x 10-5 Δt (60.0 – 0.0 sec) Rate = - (-3.50 x 10-7 mol/Lsec) Rate = +3.50 x 10-7 mole/Lsec 7 This calculation gives one the rate over a large time sampling, which is equal to the change in concentration over some unit of time. This gives one the average rate, during the first 60.0 seconds, ozone decreases at an average concentration of 3.50 x 10 -7 M per each second. However, one has no idea what is happening in terms of the change in concentration of reactant at any given instant in time. Perhaps it might be better to examine the rate of the disappearance of ozone over a shorter time interval: Calculating the rate over the first ten seconds: Rate = _ Δ[O3] = _ 2.42 x 10-5 - 3.20 x 10-5 Δt (10.0 – 0.0 sec) Rate = 7.80 x 10-7 mole/Lsec What about the rate during the last 10 seconds? Rate = _ Δ[O3] = _ 1.10 x 10-5 – 1.23 x 10-5 Δt (60.0 – 50.0 sec) Rate = 1.30 x 10-7 mole/Lsec WOW. These rates have absolutely nothing to do with one another! In fact, the later rate that we calculated is 6 times slower than the rate in the first 10 seconds of the reaction. Thus, the rate decreased over the course of the reaction. And this makes sense. Consider the factors that we mentioned earlier, such as concentrations of reactants. As the reaction proceeds, the concentrations of reactants decreases, which means that the molecules will have a tougher time finding one another which means collisions will happen less often, which in turn makes the reaction rate decrease. 8 You can plot concentrations of a reactant (or product) vs. some period of time. The curve obtained means that the rate changed as the reaction proceeded. At very high concentrations, the rate was rather quick, and the concentrations decreased very quickly per some unit of time. The longer the reaction is allowed to occur, the smaller the change in concentration. The slope of a straight line that joins any two points on the curve will give the average rate over that period of time (line b). Remember: slope = m = Δy = Δ[O3] Δx Δt The smaller the time period that you choose, the closer you get to calculating an instantaneous rate, or the rate at a particular instant in time. Think of an instant – how brief that is. On the plot, an instant occurs at a POINT on the plot. The instantaneous rate is rarely equal to the average rate. How can the instantaneous rate be determined? Pick a point on the plot and draw a line tangential to the plot. The slope of the tangent line will be equal to the instantaneous reaction rate. Basically, your Δt is so small that you are looking at your change in time being the size of the dot that you put on the plot. Remember that even though the slopes of the lines (for the disappearance of material) are negative, the reaction rates will be positive!) Determining the reaction rate can be done at any time point. A time is chosen, perhaps at 35 seconds (line d above). Then the tangent line is extended such that one can calculate (by creating a right triangle) the change in concentration per unit time. This would be the instantaneous reaction rate at 35.0 seconds. In general, the term reaction rate is synonymous with instantaneous rate as the instantaneous rate can be more meaningful than the average rate. However, many reactions that are studied are equilibrium reactions, which means that the products are actually reforming the reactants at a specific rate as well at this time point during the reaction. An easy, and less confusing way to calculate the instantaneous reaction rate is to calculate it when there is no reverse reaction to be concerned about – at a time when there is no product available to react to make more reactant. That time would be when the reaction first begins (line a). This is the initial rate, which is also an instantaneous rate taken at the moment the reactants are mixed. Under these conditions, the concentrations of the products are negligible, so the reverse reaction can be disregarded. More importantly, you know the initial concentrations of reactants and the volume of solutions mixed together. The initial rate is determined by measuring the slope of the tangential line drawn at t = 0. 9 Notice the creation of right triangles from the tangential lines. Using the extensions, changes in concentration can be determined as well as changes in time. The centerpiece of any kinetic study is the determination of the rate law, which expresses the rate as a function of reactant concentrations, product concentrations, and temperature. Any assumptions one makes about the reaction must conform to the rate law, as the rate law is experimentally determined as is fact for that particular reaction under constant reaction conditions. The rate law will be different for different reactions. Rate laws are written such that we know which species the rate depends on. Does the rate depend on all the reactants or some of the reactants. We will be focusing on reactions for which the products do not appear in the rate law. Consider the following reaction: aA + bB → dD + eE rate = k[A]m[B]n In the rate law, [A] and [B] are the concentrations of reactant A and B in moles/L (Molarity) at some particular time. The exponents, m and n are generally small positive integers (0, 1, 2) but occasionally can be nonintegral. These exponents are called the reaction orders and they have NOTHING to do with the coefficients in the balanced chemical equation. They are NOT determined by the balanced chemical reaction. The exponents are NOT derived from the balanced chemical reaction. The reaction orders can be the same as the stoichiometric coefficients but are not related in any way to the stoichiometry. The rate constant, k, is specific for a given reaction at a given temperature. It does NOT change with time as the reaction proceeds. It will depend on the particular reaction and the presence (or absence) of a catalyst. And its units will mathematically depend on the reaction order. 10 The components in the rate law can be determined by using concentrations measurements to find the initial rate. Then using initial rates from several experiments to find reaction orders, and finally using these values to calculate the rate constant. One way to determine the relationship between concentration and rate is to use the method of initial reaction rates. The same reaction is run repetitively using different concentrations each time while keeping the temperature and other conditions constant. The effect of a given reactant on the rate can then be examined by comparing runs that differ ONLY in the concentration of that reactant (the other concentrations are held constant). In studying effects of something on the reaction rate it is important to remember to examine ONE variable at a time. If you change the concentration of more than one reactant and the rate changed, which reactant caused the change? You cannot know. Change one variable at a time!! Examine the change of one variable at a time!! It is important to realize that the information in the rate law expression must be determined by experimentation. The information cannot be mathematically calculated, it cannot be deduced by the stoichiometry of the chemical equation. Before we see how reaction orders can be determined using the method of initial rates it is important to discuss the meaning of the reaction orders and important terminology. Reaction orders can be spoken of in terms of individual order, which reflect the order of the individual reactant, and then orders can be spoken of in terms of overall reaction order, which happens to be equal to the sum of the individual orders of each of the reactants. Reactant order = individual order = exponent of a reactant Overall reaction order = sum of individual orders Examining a simple reaction: aA → bB rate = k[A]m If the reaction rate is directly proportional to the concentration of the reactant A then the exponent (m) is equal to 1. The reaction is first order in A rate = k[A]1 If the reaction rate is directly proportional to the square to the concentration of A squared, then the exponent (m) is equal to 2. The reaction is second order in A. rate = k[A]2 If the rate of the reaction does not depend on the concentration of A at all, then the exponent (m) is equal to 0. The reaction is zero order in A. rate = k[A]0 rate = k (1) = k 11 Real world examples: H2 (g) + 2ICl (g) → I2 (g) + 2HCl (g) rate = k[H2][ICl] The reaction is 1st order wrt H2 The reaction is 1st order wrt ICl The reaction is 2nd order overall Concept Test: List the individual reaction order and overall reaction order for the following reactions: NO(g) + O3 (g) → NO2 (g) + O2 (g) rate = 2NO (g) + 2H2 (g) → N2 (g) + 2H2O (g) rate = (CH)3CBr (l) + H2O (l) → (CH3)3COH (l) + H+1 (aq) + Br-1 (aq) rate = Reaction orders can be fractions and they can be negative numbers. We will be focusing on reactions with positive whole number integers, but keep in mind, sometimes fractional coefficients can be terms pseudo reaction order. (e.g. a reaction with an order of 1.3 might be termed pseudo first order). It is very easy to determine or state the order of each reactant and the overall order of the reaction when the rate law is given to you. But what about when the rate law is not given to you. Then the rate law must be determined experimentally (in lab for example). But first, let’s practice some math. 12 Concept Test: Determine the exponent for each of the following: (2)x = 1 (2)x = 8 (2)x = 2 (2)x = 4 Congratulations, you just solved a rate law problem!! Calculating the exponents will be done as above. In many texts, fancy equations are used. And mathematically they work. If you prefer equations, by all means, setting up the equations like that will yield the correct exponent. However, you can also use this shorthand method to determine reaction rates. For problems in general chemistry, they will all work out to yield wonderfully nice whole number integers. In lab, you MAY see a pseudo reaction order – it really depends on how accurate you are when performing the experiment. O2 (g) + 2NO (g) → 2NO2 (g) Remember, you want to examine ONE variable at a time. That means that you need to make sure that ALL other variables are held constant. In this case, there are two variables to consider, the concentration of O2 and the concentration of NO. In order to determine the order of each reactant, we need to examine what happens to the rate when one reactant is held constant and the other changes. In experiment 1 and experiment 2, the concentration of NO is held constant while the concentration of O2 increases from 1.10 x 10-2 to 2.20 x 10-2 (the concentration is doubled) 13 What happens to the rate from reaction 1 to reaction 2? The rate increases from 3.21 x 10 -3 to 6.40 x 10-3. The rate also almost doubles (a ratio of 1.994!) So here is the problem set-up: Rate 2 = 2 Rate 1 Concentration Expt 2 = 2 Concentration Expt 1 Remember, the order is related to the concentration raised to some power And that concentration and the power are related to the rate [Concentration Ratio of Variable Changed]x = rate ratio (2)x = 2 x=1 therefore the reaction is first order with respect to O2 In experiment 2 and experiment 4 the concentration of NO is also held constant. The concentration ratio for O2 = 1.5 and the rate ratio =1.5 Therefore, we would calculate the same reactant order if we used those experiments instead. In experiment 1 and experiment 4 the concentration of NO is held constant. The concentration ratio for O2 = 3 and the rate ratio = 3 also. So again, we would get first order for O2. The only “trick” is to make sure that when you determine the ratio for the concentrations you use the same method to determine the rate ratio. Meaning, if you determine the concentration ratio using experiment 4/experiment 1 concentrations, then you MUST calculate the rate ratio using the rate of experiment 4/rate of experiment 1 (and not the reverse). Concept Test: Using the above data, determine the reactant order for NO Write the rate law Determine the overall reaction order 14 However, just because the exponents work out to be nice numbers, does not mean that the rate ratios or concentration ratios will. The following is a mathematical property of logarithms, and it should be used when determining the exponent might not be so intuitive: log Ab = b x log A Just a helpful math trick to help you determine the exponent: HINT: this may be useful for lab!! For example, (3.77)x = 53.58 x (log 3.77) = log 53.58 x = log 53.58 log 3.77 x = 1.729 0.5763 x = 3.000 NOTE!!!: log 53.58 log 3.77 does NOT equal log 53.98 3.77 The rate constant, k, can then be determined for a particular reaction using the reaction rates and concentrations. However, the units for k will depend on the reactant order. Since it is an exponent, some of the units will be squared, or even cubed! The following chart can be used, but you should be able to mathematically deduce the units for k using the units in the equation. Error in second line 15 The rate laws that you have derived so far really do not have a time component. You have examined average rates and instantaneous rates and changes in concentration at a particular instant in time. However, by creating an integrated rate law you can consider the time factor and answer questions such as “how long until my reactant is used up?” or “what is the concentration of reactant after some number of minutes?” Using the average rate you assume that the concentration is changing at the same rate the entire time – remember back to your homework when you calculated the Molarity of HNO3 after 3 minutes and you all got really weird numbers (like 60 M). That was because you were making the assumption that the reaction proceeded at that rate the ENTIRE time. We now know that is not true. But how to we map the rate along its course – how to we deal with zero order, first order, and second order reactions, which each mean different things? Let’s examine each reaction order one at a time. For a first order reaction, such as the following: 2N2O5 (g) → 4NO2 (g) + O2 (g) rate = k[N2O5] general rate equation: rate = k[A] the concentration of N2O5 is going to decrease with time according to the equation: [A] = [A]0e-kt where [A]t is the concentration of substance after some time where [A]0 is the initial concentration Rearranging the equation will give you the following: ln [A]t = -kt [A]0 which can be mathematically rearranged to: ln [A]t – ln[A]0 = -kt ln[A]t = -kt + ln[A]0 y = mx + b A plot of ln[A]t vs. time (y vs. x) will generate a straight line for a FIRST ORDER reaction 16 When a reaction is first order with respect to the reactant, taking the ln of the concentrations and plotting the data will give you a straight line, such that the slope will be equal to –k. A graph using the natural log will result in a straight line for a first order reaction. YES, these equations will be given to you. Just be familiar that you can turn non-linear mathematical relationships into linear relationships from which you can determine k!! Therefore, using the plot, or the equation for the line and the concentration data and time you can determine k. You do not have rate data – notice you just have time and concentration data. From the plot, the k value can be determined and then using the initial concentration and some amount of time you can determine – accurately exactly how much reactant you have used up. If you know exactly how much reactant you have used up you can calculate exactly how much product is formed The simplest second order reaction is one in which the rate depends on the square of a single reactant (how we will deal with this type of reaction) rate = k[A]2 For second order reactions, the concentration of this type, the concentration of A is going to vary with time according to the following equation: 1 = kt + 1 [A]t [A]0 A plot of 1 vs. time will generate a straight line for a [A]t SECOND ORDER reaction Now when this data is plotted, the slope = +k. Again, you can examine the plot and the straight line and generate the k value. You can also use concentration data (which would be given) and calculate k. Or if given k you can examine changes in concentration over time! And again, if you have the k value from the plot, and given initial concentration data, you can now calculate the exact amount of reactant left after some amount of time has passed. 17 When the rate does not depend at all on the reactant concentration we say we have a zero order reaction, such that it obeys the following rate law: rate = k[A]0 rate = k In order to generate a plot which we can use to determine k, we will use the following equation: [A]t = -kt + [A]0 A plot of [A]t vs. time will generate a straight line for a ZERO ORDER reaction Again, the slope of the line will be equal to –k. If the rate law is unknown, you will get a lot of time and concentration data. Plot the data. See what you get. And then you will have to mathematically manipulate it. If you plot the data as is and get a straight line, you are looking at zero order for the reactant. If you plot the data and only get a straight line when you lake ln[X] then the reactant is first order. If you plot the data and have to plot 1/[X] in order to get a straight line, then the reactant is second order. See, it really is a lot of guesswork – trial and error! Only by actually investigating the mathematical relationships of the data that you acquire can k and the rate law be determined. Half life studies can also be done by examining how long it takes for half the material to be consumed. Most of you have probably heard of half-lives when dealing with nuclear chemistry and how long does it take for a region that has radioactive material to become safe again. Or, how long will a radioactive species be around. Simply speaking, the half-life for any material, is the amount of time it takes for ½ of the material to disappear. If only half is disappearing over a chunk of time, you may have to wait decades or centuries for material to disappear to immeasurable levels. Each reaction order has its own mathematical expression to determine the half-life. Check out some of the following half-lives below: 18 Chemical Species 198Au 14C 37Ca 60Co 137Cs 53Fe 220Fr 3H 131I 37K 85Kr 16N 32P 239Pu 226Ra 222Rn 90Sr 99Tc 232Th 233U 235U 238U Half-Life 2.69 days 5730 years 175 millisec 5.26 years 30.23 years 8.51 min 27.5 sec 12.26 yr 8.07 days 1.23 sec 12.4 hrs 7.2 sec 14.3 days 2.44 x 104 yrs 1600 yrs 3.82 days 28.1 yrs 2.13 x 105 yrs 1.4 x 1010 yrs 1.62 x 105 yrs 7.1 x 108 yrs 4.51 x 109 yrs 19 Temperature has a major effect on reaction rate. We heat food to cook it, and the higher the temperature the faster the food cooks. Before the days of air-conditioning, photographers observed that the time needed to develop film was approximately halved whenever the temperature increased 10oC. From this observation, the “photographer’s rule of thumb” was developed: “For every 10oC rise in temperature, rates of reactions will approximately double. A reaction that has a certain rate at 20oC would go twice as fast at 30oC, four times as fast at 40oC, and eight times as fast at 50oC. Many other reactions follow the same general rate increase pattern, BUT it is an estimate of approximate changes in rate. Not all reactions do! If you collect reaction time data for the same reaction (same concentrations) just run at different temperatures, you will see that in the rate law, it is the value of k that changes. In fact, as the temperature increases, k increases. An equation (of course) has been derived to examine the relationship between temperature and the rate constant. Known as the Arrhenius equation: k = Ae-Ea/RT where Ea is the activation energy R = 8.314 J/molK A = frequency factor that takes into account the orientation needed for correct collisions So, we know that the rate changes when concentrations change (you investigated this in lab), and we have learned that rates will also change as the temperature changes (if we leave that milk out of the fridge it spoils a LOT faster than if we remember to put it in there!). But by now, we know that just making these observations is not enough Now its time to learn the WHY behind the rate changes. There are two theories on WHY the rate increases, and they are complimentary to one another. You should be familiar with both of them. For those of you going on to organic, the information from here to the end of the term is very important – so pay attention!!! Collision theory views reaction rates as the result of particles colliding with a certain frequency (how often) and with a certain minimum energy on order for the reaction to occur. Transition state theory offers a close-up view of how the energy of the collision allows the reactant to be converted into product. 20 Collision theory explains why the concentrations in the rate law are multiplied together instead of added. Imagine if you have two molecules of A and two molecules of B. In order for the chemical reaction to occur, A and B must meet. A1 can combine with B1 or A1 can combine with B1. A2 can combine with B1 or A2 can combine with B2. Thus, with 2 of each particle present, there are 4 possible combinations. If another A molecule is added, the number of possible combinations is 6. A1 B1 A2 B2 A3 A1 with B1 A1 with B2 A2 with B1 A2 with B2 A3 with B1 A3 with B2 Thus, the collision model is consistent with multiplying concentrations, as the number of particles and their possible collisional combinations is a product – not a sum. Increasing the temperature of a reaction increases the average kinetic energy of the molecules, making them move faster and thus, increases their collisional frequency. We have previously talked about how temperature affects molecules speeds. In class, each of you is taking notes, some of you are shifting in your seats, some of you get up to sharpen your pencils, and some of you are sleeping. There is movement. However, you are not really colliding with one another; sometimes maybe you accidentally kick your neighbor, or reach over and tap them to ask a question. However, if I lit a fire in the room and locked you all inside, chances are, you will definitely be 1.) moving faster and with more energy, and 2.) colliding and running into one another more often! But how often molecules collide is not the only factor to consider. Because it is known that at 20oC and 1 atm the molecules in 1 mL of gas experience about 1027 collisions per second. And at that rate, all reactions would be over instantaneously if all that mattered was that they run into one another. In fact, the vast majority of collisions result in the molecules bouncing off one another, much like billiard balls in a game of pool or like bumper cars at the local fair. Every reaction that occurs has an energy threshold that must be met when molecules collide in order for them to react. A great analogy to this is an athlete who must jump over a hurdle or a high bar in order to complete the “race”. The minimum energy needed for the collisions to mean that the molecules react instead of bouncing off one another is called the activation energy (E a). This is the energy required to activate the state from which reactant bonds can be broken and product bonds formed. At any given temperature, there are molecules with many different kinetic energies, thus when they collide they collide with a wide range of collisional energies as 21 well. According to the collision theory, ONLY those molecules that collide with enough energy to exceed or meet the activation energy will react to form product. Increasing the temperature makes the molecules move faster, but more importantly, increasing the temperature increases the number of collisions that have enough energy to exceed the activation energy which increases the rate at which product is formed. The activation energy can be seen on an energy diagram which shows the conversion between reactants and products. We previously examined energy diagrams when we looked at exo and endothermic reactions. The “hump” between the reactants starting energy and the products that are formed is the amount of energy needed for the reaction to occur. All reactions need a certain amount of energy in order to overcome the hump. Even spontaneous reactions have this activation energy. There are two activation energies present on any diagram. As chemical reactions are reversible, there is the activation energy required for the forward direction (as written, from reactants to products) and there is an activation energy required to convert the products back to the reactants. Notice that one of the activation energies is larger than the other – and it depends on whether the final energy state is lower or higher than the initial energy state. You should be able to label an energy diagram with all the components pictured below and define/explain what is occurring during the reaction. energy Endothermic Reaction products Ea reverse Efinal > Einitial reactants Ea forward time 22 Exothermic Reaction reactants Ea forward Efinal < Einitial Ea reverse products time The activation energy value will change from reaction to reaction. Thus, the value of E a and the temperature will affect exactly how many molecules have enough energy to be converted from reactants to products. The rate of the reaction will depend on how long it takes for the molecules to have sufficient energy to make it over the hill to become products. If not many molecules make it, then the amount of product produced will be small. If many molecules make it into the product form, then the amount of product produced can be substantial. Notice that the height of Ea forward and Ea reverse are different. The smaller the Ea the faster that reaction will proceed comparatively speaking. That means that for an endothermic reaction, the reverse reaction will occur faster than the forward reaction. And for an exothermic reaction, the forward reaction will occur faster than the reverse. Larger Ea => smaller k values => slower reaction rates Smaller Ea => larger k values => faster reaction rates There is a final component for the collision theory, and that is molecular structure. Even though there are millions upon millions of collisions occurring per second, not very many occur with substantial energy in order to exceed the activation energy to become products. In addition to having the correct amount of energy, the molecules MUST collide in such a way that is favorable for the reaction to occur – meaning they must come together like pieces in a puzzle. This is often termed correct orientation. The molecules must be orientated in such a manner that the region of the molecule that is undergoing the reaction meets with the correct region of the other molecule so that the reaction can actually take place. Much like putting a puzzle 23 together. You may have the LAST two pieces of the puzzle in your hands, but they do not fit together in some haphazard way do they? No, the little indentations and outcroppings only fit together and lock into place in one way. The same is true with molecules. They fit together in a specific way in order for the reaction to take place. The puzzle piece can only fit ONE way in order to complete the picture The same is true of molecules, they must meet in a certain way in order to react and create the final picture (the products) The probability of the molecules having the correct energy AND the correct orientation makes investigating reaction rates a pretty complicated thing. In fact, for one chemical reaction [ NO(g) + NO3 (g) → 2NO2 (g)] , only 1 out of every 167 collisions results in the correct orientation and energy that will lead to the creation of product. For some chemical reaction, orientation is not the all important factor in determining if the reaction will take place, the frequency and activation energy are more important. For other chemical reactions, especially biochemical reactions, orientation is the major bottleneck in determining if the chemical reaction will occur. In fact, for some biochemical reactions, orientation is so important that only 1 in a million molecules are correctly orientated and have sufficient energy to react. Thus, it is a GOOD thing that there are 1027 collisions per second! And it makes our functioning bodies quite amazing! The transition state theory focuses in on this activation energy, what it is, why it is important, and what exactly is occurring or what exists in the transition state. For those of you going on to organic chemistry, you will talk a lot about these “activated complexes” as organic reactions proceed. When molecules are moving at a great speed towards one another they have kinetic energy. As molecules get closer to one another, their speed slows and their kinetic energy is converted into potential energy. Their electron clouds begin to overlap and if they do not have enough motion to continue to push them towards one another, the electron clouds cause the two molecules to repulse one another and they “bounce away”. Just like two magnets that approach one another with the same poles put together. BUT, if their kinetic energy is great enough to overcome the 24 electron cloud repulsion, then the molecules can undergo the chemical reaction. The nuclei of the opposite atoms are attracted to the electrons in the other atom/molecule (remember back to electron electron repulsion – bounce away, nuclei nuclei repulsions, and nuclei electron attractions - react??) Now you understand why those concepts are so important, they help dictate whether the reaction will occur! As the opposite nuclei attract the other electron clouds, orbitals begin to overlap, bonds break and new bonds form. The molecules gradually almost in a step by step manner change forms, morphing from individual unique molecules into some new form, creating new molecules which we call products. If you could watch the process in slow motion you would see that the transformations that occur are neither reactant nor product, but a mutant combination of the two, called a transitional species. The transitional species is/are very unstable and are impossible to analyze (yet scientists would really like to capture the molecules along their reaction paths). This species is the species that exists at the very top of the energy diagram, and it is called the transition state or the activated complex. The transition states cannot be analyzed, so they are actually an attempt to explain what happens in the reaction on paper. We can make very good “guesstimates” of the reaction path and are determined by chemical reasoning, chemical knowledge of reacting species, and by studying analogous, and more stable species. Reaching the transition state is no guarantee that the reaction will proceed to the products. It is the tippy top of the plot. It is the top of the hill. As far as energy is concerned, it is a fine balancing act. Think of a see-saw. When you are on the see-saw with another person and you balance exactly on the pivot point, at any moment in time you could go up or you could be the one that goes down. The same is true with chemical reactions. The reactants may not have quite enough energy to make it over the hill and they fall back apart (decompose) into individual species, or they may make it over the hill and slide to the product side (combination). Transition state theory is based on the idea that every reaction is reversible. And that every step in a reaction goes through its own transition state. At the top of the reaction diagram, oftentimes a picture or figure of what the molecular species looks like is drawn. 25 CO and NO2 react to for CO2 and NO The transition state or activated complex shows the two molecules “linked” or appear as if they are bonded together. If the OC bond breaks, the reactants will be reformed. If the NO bond breaks, then the products will be formed. Examining what the transition state is, is key into understanding how the molecules come together to create the products. Obviously the products formed were not OCN and O 2 therefore, NO2 lost and oxygen and CO gained it. Thus, we know what atoms must come together, we know what bonds broke, we know what bonds were formed, and we know the shapes and structures of all the molecules. Basically, monitoring the reaction, all the permutations and shape and steps along the way is what scientists study when they examine reaction mechanisms. This is a MAJOR component of organic chemistry – where you will spend hours upon hours of learning reaction mechanisms and exactly HOW species come together to form products. There are many generalizations that can be made about how species react, such that you will even be able to go so far as to predict the products of the reaction. But for now, this is the introduction to all of that. A reaction mechanism is a series of steps that a reaction will undergo as it changes from reactants to products. Sometimes it can be as simple as A→ B through a transition state complex and other times it can be an exciting series of umpteen steps each again, with its own transition state. Remember, any time species come together to make something new, there is a transition state complex. 26 Imagine the following reaction: 2A + B → E + F Perhaps the mechanism is: A + B → C C + A → D D → E + F So, in English, reactants A and B react to form a compound, C But C can re-react with A forming another compound D D is able to decompose into the final products that we observe, E and F We can verify that the above steps yield the overall reaction just like we did for Hess’ law. Do the reactions given yield the known equation, indicating the correct ratio of reactants and products AND yield only the stated reactants and products in the known chemical reaction? A + B + C + A + D → C + D + E + F Again we can cross of species that appear on both sides of the yield arrow, just as we did in Hess’ law and for balancing redox reactions, AND combine species that are the same 2A + B → E + F The pathway that we propose gets us from reactants to products But what are these species, C and D, which appear in our mechanism but not in our overall equation? Where did they come from? These species are known as reaction intermediates. The intermediate is a species that is formed in the chemical reaction but is used up completely during the course of the overall reaction. They do not appear in the overall chemical reaction (notice that the intermediates appear on both sides of the chemical reaction, thus they cancel out) but they are necessary for the reaction to occur. They are not as stable as the reactants or products, but they are more stable than transition state complexes and thus can oftentimes be “trapped” and analyzed. In order for a transition state to be analyzed, the conditions must be such that the intermediate can be stabilized. In an energy diagram, this would be shown as an energy well, termed a potential energy well. If the transition state is the peak of the hill, then the intermediates are little ledges on the way down to the formation of the product. 27 The reactants “fall” from the transition state peak and hit a well, where the intermediate is stable. Notice the new transition state that follows the well, whereby the intermediate must overcome an activation energy in order to move to the next well. The individual steps, which together make up the proposed reaction mechanism, are called elementary reactions. Each describes a single chemical event, such as a combination reaction or a decomposition reaction. The elementary steps are as simple as it can get. The elementary steps can be described by the number of chemical components that are involved. For a combination reaction, two things come together so the step is described as bimolecular. In a decomposition reaction, one species breaks down into other species, and is termed a unimolcular reaction (uni can mean 1, think of unicycle). The overall reaction: 2O3 (g) → 3O2 (g) (1) O3 (g) → O2 (g) + O (g) (2) O3 (g) + O (g) → 2O2 (g) (1) the decomposition of ozone (O3) contains ONE species and is unimolecular (2) the combination of ozone with oxygen contains TWO species and is bimolecular Termolecular steps are rare, think of not only the energy needed for the reaction to occur but now THREE species must come together with the correct orientation to react. SKIP!! NOTE: Rate laws for elementary steps are different than the rate laws for the overall chemical reaction. The rate laws for the elementary steps CAN be deduced from the stoichiometry. Thus 28 the rate law for (1) = k[O3] and the rate law for (2) = k[O3][O]. This ONLY holds true for elementary steps in a mechanism. All reactions do not have the same rate. The same holds true for the elementary steps. They too are chemical reaction, and as such, each step has its own rate. If each step along the way depends on the previous step, then how fast each step proceeds depends on how fast the previous step is. In some manner, a step-wise reaction is like a relay race. Does every runner on the relay team run exactly the same speed during the race? If you lose the relay by 1 second, whose fault would that be? The fastest runner on the team? The second fastest? Or the slowest? Right, the slowest. The slowest runner, or the slowest step in the mechanism is the bottleneck to the entire rate of the overall reaction. The reaction can only proceed as fast as the slowest step. Because the slowest step determines the overall rate of the reaction, the rate law for the slowest step is the rate law for the overall reaction! Consider the reaction between CO and NO2 which forms CO2 and NO CO (g) + NO2 (g) → CO2 (g) + NO (g) IF the reaction occurred in ONE step, then the elementary step would consist of CO and NO2 combining directly to form CO2 and NO As the elementary step, that would make the rate law: rate = k[CO][NO2] However, the known rate law is: Rate = k[NO2]2 This means that the mechanism is not a simple combination reaction! The elementary step, the rate determining step MUST have [NO2]2 It MUST The actual mechanism is as follows: (1) NO2 (g) + NO2 (g) → NO3 (g) + NO (g) SLOW (2) NO3 (g) + CO (g) → NO2 (g) + CO2 (g) FAST rate (1) = k[NO2]2 rate (2) = k[NO3][CO] Such that the overall sum indeed adds to give the target equation and such that the SLOW step determines the rate law for the overall reaction. As the rate law for the elementary steps can be written based on the stoichiometry of each reaction, we see that the slow step DOES describe the known rate law for the reaction. 29 You can never prove that a mechanism represents the actual chemical change. If the evidence supports it, it can be considered a valid mechanism. It is just a proposal of the steps that a reaction will undergo. You can disprove mechanisms. If the slow step in the mechanism does NOT describe the known rate law, then the mechanism cannot be correct. If you can trap an intermediate along the way, and that trapped intermediate is identified and is NOT part of your mechanism, then your mechanism is no good. Some rules that must be followed for reaction mechanisms: 1.) the elementary steps must add up to the overall target equation 2.) the elementary steps must be physically reasonable (either unimolecular or bimolecular) 3.) the mechanism MUST correlate with the rate law There are many reasons that one might want to speed up a chemical reaction. Heating a reaction up can speed up a reaction, however, if the substance is not stable to high temperatures, then adding heat may not be a good idea. Also, heat costs money. Perhaps there is another way to speed up a reaction. You can use a catalyst, a substance that increases the rate of the chemical reaction without being consumed in the chemical reaction itself. Because they are not consumed, you only need a small amount of catalyst present to speed up the reaction. Each catalyst has its own specific way of functioning; however, in general, they speed up the rate of reaction by lowering the activation energy. If the activation energy is lowered, then more molecules t hat collide will have that sufficient amount of energy to be converted from reactants to products. By lowering the activations energy (Ea) this makes the rate constant larger which in turn the rate higher. More reactants are converted over into products per unit time. A catalyst speeds up both the forward and reverse reaction rates since the activation energy is lowered it works on BOTH directions of the chemical reaction. A reaction that has a catalyst produces the SAME amount of product that would have been produced without the catalyst; it just produces that amount faster. It is the SAME amount, only the rate is changed. The activation energy is lowered by giving the reaction (the reactants) a different mechanism for the reaction to occur. This different mechanism has a lower Ea value. In the figure below, ozone and oxygen molecules combine to form O2. The reaction can proceed via one mechanism which must overcome activation energy of 17.1 kJ. Or, it can go through a two step mechanism (remember, each step has its OWN transition state) which takes 2.1 kJ of energy for the first step and 0.4 kJ of energy for the second step, which is a total energy required of 2.5 kJ. This is a significantly lower amount of energy needed for the reaction to occur. Notice in the catalyzed reaction, which the presence of Cl exists with the reactants, and at the end, Cl is still present, chemically unchanged. 30 31