TTH PCR Protocol (Hot Start)
advertisement

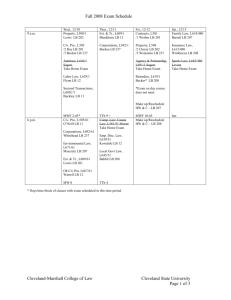
TTH PCR Protocol (Hot Start) 1. This protocol is based off of using Applied Biosystems’ Gene Amp XL PCR Kit (Part #: N808-0193). 2. Each well should contain: 1µl of 100ng/µl DNA 1µl of 3.2pmol/µl primer #1 or 1.25µl of 100ng/µl primer #1 1µl of 3.2pmol/µl primer #2 or 1.25µl of 100ng/µl primer #2 3. Next, make two mixes. One is the initial Master Mix, and the other is the TTH Master Mix which will be added after the reactions have been cycled with heat. 4. Master Mix: Per rxn: 3µl 3.3X buffer 2µl dNTPs 1.2µl MgOAc 1.3µl di water 5. Add 7.5µl Master Mix to each well. 6. Go ahead and prepare the TTH Mix, but don’t add it yet. 7. TTH Mix: Per rxn: 4.5µl 3.3X buffer .5µl enzyme (add to mix just before adding mix to sample) 9.75µl di water 8. Quick spin you reactions to pull them to the bottom. 9. Begin cycling the reactions (see cycling conditions below). 10. After the reactions have been heated at 95C for 2 minutes, pause the cycler and add 14.75µl of the TTH Mix to each sample. You may want to pull out the plate and tap it on the counter so that the liquid goes to the bottom. 11. Restart the cycler and allow it to finish. 12. Run reactions on a gel (5µl each), and proceed with SAP clean-up or gel purification. Cycling Conditions: 95°C for 2 minutes 95°C for 2 minutes 95°C for 5 minutes 30 cycles of: 95°C for 1 minute 55°C for 1 minute 65°C for 5 minutes 72°C for 10 minutes Hold at 4°C