hep26394-sup-0004-suppinfo
advertisement

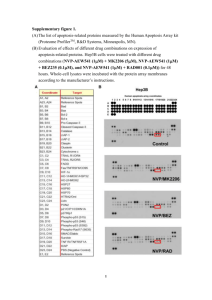
Wang et al Hedgehog Signaling Regulates Autophagy SUPPORTING INFORMATION SUPPORTING EXPERIMENTAL PROCEDURES Cell Culture and Reagents. Three human hepatocellular carcinoma cell lines (HCC cell lines), including Huh7, Hep3B and HepG2 cells were utilized in this study. Cell lines were cultured in Minimum Essential Media (GIBCO) with 10% FBS at 37°C in a 5%CO2 atmosphere. SAG, Purmorphamine (Pur), GANT61, oxaliplatin, U0126 and zVAD-fmk were obtained from Santa Cruz Biotechnology, recombinant human sonic hedgehog N-terminus (Shh) ligand from R&D systems, carbamazepine, 3-methyladenine (3-MA), E-64d, pepstatin A, chloroquine (CQ) and Hoechst 33342 from Sigma-Aldrich, and GDC-0449 from Selleckchem Bio. Stock solutions were prepared in H2O, dimethyl sulfoxide (DMSO) or ethanol as appropriate and stored at 20°C. Antibodies against Shh, Patched, Gli1, LC3II, p62, Beclin-1, Bcl2, caspase-3, 7, 8, 9, PARP, p53, DNMT1, DNMT3a, NF-κB, MEK and ERK were purchased from Cell Signaling Technology, and against Smo, Bnip3 were from Santa Cruz Biotechnology. Pro-apoptosis Bcl-2 family antibody kit, pro-survival Bcl-2 family antibody kit and autophagy antibody kit were from Cell Signaling Technology. WST1 and Annexin-V-Fluos Staining Kit were from Roche Molecular Biochemicals. Treatment with Drugs/Chemicals. The following drugs or chemicals were applied as indicated in the Figure legends: SAG (0.5 µM for 24 or 48 hours), Pur (10 µM for 24 or 48 hours), Shh (0.4 µg /mL for 24 or 48 hours), GANT61 (range 0- 20 µM for up to 72 hours), GDC-0449 (20 µM for 24 or 48 hours), CBZ (200 µM), oxaliplatin(20 µM), 3-MA (5 mmol/L), E-64d/pepstatin A (10 µg/ml), U0126 (10 µM ), CQ(20 µM) and zVAD-fmk(20 µg /mL). GFP-LC3 Puncta-Formation Analysis. Cells were transfected with a green fluorescence protein conjugated LC3 protein (GFP-LC3) expression plasmid using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) and then subjected to treatment with Hh agonist or antagonist for 24 hours. GFP-LC3-positive (>3 punctate staining sites per cell) cells were defined as an indicator of autophagy under inverted fluorescence microscope (Olympus IX70). 1 Wang et al Hedgehog Signaling Regulates Autophagy Gli-Luciferase Assay. Huh7, HepG2 and Hep3B cells were plated in triplicate on 6-well plates and transiently transfected with 4 µg Gli-luciferase reporter (Signosis, Inc) and 0.2 µg pRL-TK (Renilla Luciferase driven by TK promoter, Promega) using Lipofectamine 2000 (Invitrogen). Six hours post-transfection, cells were treated with Hh agonists or antagonists for another 24 hours, and harvested using the dual-luciferase reporter assay kit (Promega Corporation, Madison, WI) according to the manufacturer’s protocol. Luciferase activity was measured by a Centro XS3 LB 960 microplate Luminometer (Berthold Technologies) and the data were normalized to the activity of a Renilla luciferase internal control. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR). Huh7, HepG2 and Hep3B cells were treated as indicated in the figure legends. Total RNA was extracted with TRIzol® plus RNA purification kit (Ambion, Grand Island, NY) and reversetranscribed to cDNA using SuperscriptTM II reverse transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. cDNA samples were used for real-time PCR analysis in triplicate using QuantiFast SYBR Green PCR kit (Qiagen) on Bio-Rad C1000 Thermal Cycler. Primers specific for Gli1, Ptch1, Bnip3 and Actin were from Qiagen. Target gene levels were presented by a comparative CT method with 2- ΔΔCt and standardized by the internal control gene Actin. Western Blotting and Immunoprecipitation Assays. Cells were washed twice with ice-cold PBS and lysed in a lysis buffer containing 50 mM HEPES, 1 mM EDTA (pH 8.0); 1 tablet of Roche protease inhibitors cocktail was added per 10 mL buffer. After sonication on ice, the cell lysates were centrifuged at 12,000g for 5 min at 4°C and the supernatants were collected for Western blotting assay. The protein concentration was measured with the Bio-Rad Protein Assay Kit (Bio-Rad, Hercules, CA). After boiling for 5 min in the protein loading buffer with 2mercaptoethanol, the samples were separated on a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel and then transferred onto the nitrocellulose membrane (BioRad). The membranes were blocked in PBS with 0.1% Tween 20 (PBS-T) containing 5% nonfat milk for 1 h. Blots were incubated with different primary antibodies (at appropriate dilutions) in PBS-T containing 5% nonfat milk at 4°C overnight. After wash with PBS-T, the 2 Wang et al Hedgehog Signaling Regulates Autophagy membranes were incubated with secondary antibody (IRDye conjugated IgG, LI-COR) in PBS-T containing 5% nonfat milk for 1 hour and the signals were then determined with Odyssey Imaging System (LI-COR). For immunoprecipitation, the cells in 100-mm dishes were lysed in 1 ml of the whole-cell extract buffer containing 50 mM Tris-HCl pH7.6, 150 mM NaCl, 0.1 mM EDTA, 1% NP-40, 1.0 mM DTT and protease inhibitors. 500 μl of cell lysates were used for immunoprecipitation with specific antibodies. In brief, cell lysate was precleared with 30 μL protein A/G-plus agarose beads (Santa Cruz) by rotation at 4°C for 1 h. The supernatant was obtained after centrifugation 5000 rpm at 4°C for 5 min. The precleared supernatants were incubated with 2 μg antibody by rotation at 4°C for 4 hour, and then incubated with 20 μl protein A/G-plus agarose beads by rotation at 4°C overnight. The samples were collected by centrifugation at 8000 rpm at 4°C for 3 min, followed by wash with beads wash solution (50 mM Tris-HCl pH7.6, 150 mM NaCl, 1 mM EDTA and 0.1% NP-40) and then suspended in 2×SDS-PAGE sample loading buffer. After boiling for 5 min, the samples were subjected to SDS-PAGE and Western blotting with specific antibodies. Transmission Electron Microscopy. Huh7 cells were treated with 20µm GANT61 for 48 hours and harvested by trypsinization, washed twice with PBS, fixed with ice-cold 4% glutaraldehyde in 0.1 mol/L cacodylate buffer, postfixed in osmium tetroxide, and then embedded in Epong. A 1.0-µm-thin section was cut, stained with methylene buffer ArumeII, and examined under an electron microscope. Monodansylcadaverine (MDC) Staining. Huh7 cells were seeded into 96-wells plates (5×104 cells/well) and treated with indicated Hh pathway agonists or antagonists. The cells were then washed with cell-based assay buffer (Cayman Chemical), incubated with 100 μl of Cell-Based MDC solution (Cayman Chemical) for 10 min at 37°C, and examined under a fluorescence microscope (Olympus IX70). Plasmid or siRNA Transfection. Bcl-2 overexpression plasmid (Addgene), Bnip3 overexpression plasmid (Origene, Rockville, MD) or Bnip3 specific siRNA (Santa Cruz Biotechnology) was transfected into Huh7 cells by using Lipofectamine 2000 (Invitrogen, 3 Wang et al Hedgehog Signaling Regulates Autophagy Carlsbad, CA) as manufacturer’s instruction. After 6 hours transfection, cells were treated as indicated in figure legends. Cells were analyzed for apoptosis and the cell lysates were obtained for Western blotting and immunoprecipitation. Culture Plate Colony-Formation Assay. 1×103 Huh7 cells were plated into 100-mm dishes and treated with SAG, Pur, GANT61 or GANT61 plus 3-MA for 14 days to allow colony formation. Colonies were stained with 0.1% crystal violet (Amersco, Solon, OH) in 50% methanol and 10% glacial acetic acid for counting. Cells Viability WST1 Assay. The cell proliferation reagent WST-1 was used for quantitative determination of cellular proliferation (Roche Molecular Biochemicals, Germany) according to the manufacturer’s instruction. In brief, 4×103 cells were plated in triplicate into 96-well microliter plates in 100μl culture medium containing 10% FBS. After specific treatments, the cells were incubated for additional 2 hours in the presence of 10 μl/well WST-1 labeling mixture at 37°C and 5% CO2. The absorbance of the samples was measured at 450 nm using an automatic ELISA plate reader (VersaMax Microplate Reader). Hoechst 33342 staining for apoptosis assessment. Huh7 cells were seeded into 6-wells plates and cultured as described above. After indicated treatments, the cells were washed twice with PBS and then stained with 10 μg/mL Hoechst 33342 (Sigma-Aldrich, USA) for 40 min at 37°C. Morphologic changes in apoptotic nuclei were observed and photographed under the fluorescence microscope with emission wavelength at 460 nm and excitation wavelength at 350 nm. Apoptosis was defined as the presence of nuclear condensation and DNA fragmentation on Hoechst staining. Flow Cytometry Analysis of Annexin-V/Propidium Iodide Staining. Detection of apoptosis in HCC cells by Annexin V/PI staining was performed with Annexin V-FLUOS Staining kit (Roche Molecular Biochemicals, Germany) according to the manufacturer’s instructions. Briefly, 1×106 Cells were harvested and centrifuged at 200×g for 5 min. After washing with PBS, the cell pellets were resuspended in 100 μl Annexin-V-FLUOS labeling solution containing of 2 μl Annexin-V-FLUOS labeling reagent and 2 μl Propidium iodide solution for 15 min in the dark. 4 Wang et al Hedgehog Signaling Regulates Autophagy After resuspension in 0.5 ml incubation buffer, the cells were immediately analyzed on a FACScan Flow Cytometer (BD LSRII). Early stage of apoptosis was indicated as Annexin V (+), PI (-) % cells and late stage of apoptosis was as Annexin V (+), PI (+) % cells. Xenograft tumor study. Male SCID (severe combined immune deficiency) mice were housed under standard conditions. Each mouse was subcutaneously inoculated into flank with 1x107 Huh7 cells suspended in a total volume of 100 µl phosphate buffered saline (PBS). The inoculated tumor cells were allowed to grow for 1 week before drug treatment. The SCID mice were randomized to three groups, and treated with vehicle only, GANT61 (50 mg/kg) and GANT61 (50 mg/kg) combination with 3-MA (10 mg/kg) by intraperitoneal (i.p.) injection every other day for 4 weeks. The resulting tumors were measured for the longest diameter (a) and the shortest diameter (b), and the tumor volumes were calculated as follows: V=a × b2/2. Data were presented as tumor volume (mean ± SD). A portion of each tumor was used for hematoxylineosin (H&E) staining, western blotting analysis for LC3II and caspases, and immunofluorescent staining for LC3II. Statistical Analysis. Quantitative data were presented as mean ± standard deviation from a minimum of 3 replicates. Statistical difference between groups was analyzed by SPSS 13.0 statistical software with one-way analysis of variance or Student t test. A p-value <.05 was considered as statistically significant. 5 Wang et al Hedgehog Signaling Regulates Autophagy SUPPORTING FIGURE LEGENDS SUPPORTING Figure S1. Inhibition of Hh signaling induces autophagy. (A) MDC (monodansylcadaverine) staining. Huh7 cells grown in 96-wells plates were treated with vehicle, SAG (0.5 μM), Pur (10 μM), Shh (0.4 μg/ml), GANT61 (20 μM) or GDC0449 (20 μM) for 24 hours. MDC staining was performed and the cells were examined under a fluorescence microscope. (B) Transmission electron microscopy showed autophagosomes and autophagolysosomes (arrow indicated) in GANT61-treated Huh7 cells. SUPPORTING Figure S2. GANT61 does not increase ATG gene expression. Huh7 (A), Hep3B (B) and HepG2 (C) cells were treated for 48 hours with 0.5 μM SAG, 10 μM Pur, 0.4 μg/ml Shh, and 20 μM GANT61, respectively. The expression of ATG genes (ATG 3, 5, 6, 7, 12) was examined by Western blotting. SUPPORTING Figure S3. Western blotting for NF-κB (A), p53 (B) and DNAmethyltransferase 1 and 3a (C) in Huh7 cells treated with 0.5 μM SAG, 10 μM Pur or 20 μM GANT61, respectively, for 48 hours. 6