List of Approved FDC Drugs till November 14 1. Each gram of
advertisement
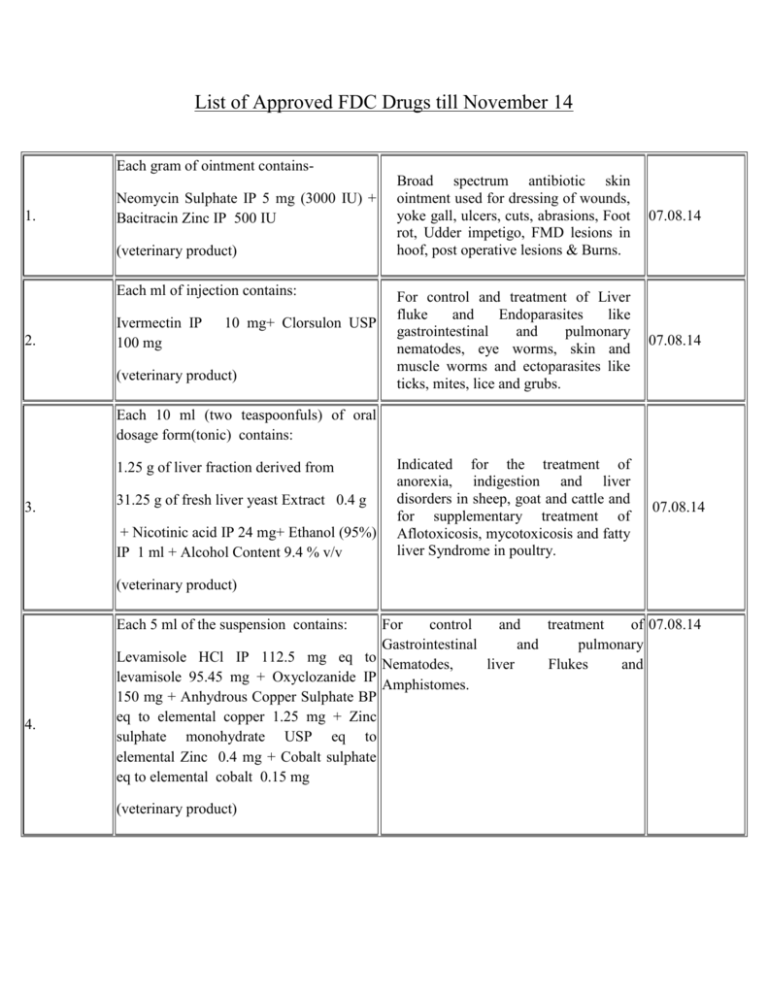
List of Approved FDC Drugs till November 14 Each gram of ointment contains1. Neomycin Sulphate IP 5 mg (3000 IU) + Bacitracin Zinc IP 500 IU (veterinary product) Each ml of injection contains: 2. Ivermectin IP 100 mg 10 mg+ Clorsulon USP (veterinary product) Broad spectrum antibiotic skin ointment used for dressing of wounds, yoke gall, ulcers, cuts, abrasions, Foot rot, Udder impetigo, FMD lesions in hoof, post operative lesions & Burns. 07.08.14 For control and treatment of Liver fluke and Endoparasites like gastrointestinal and pulmonary nematodes, eye worms, skin and muscle worms and ectoparasites like ticks, mites, lice and grubs. 07.08.14 Indicated for the treatment of anorexia, indigestion and liver disorders in sheep, goat and cattle and for supplementary treatment of Aflotoxicosis, mycotoxicosis and fatty liver Syndrome in poultry. 07.08.14 Each 10 ml (two teaspoonfuls) of oral dosage form(tonic) contains: 1.25 g of liver fraction derived from 3. 31.25 g of fresh liver yeast Extract 0.4 g + Nicotinic acid IP 24 mg+ Ethanol (95%) IP 1 ml + Alcohol Content 9.4 % v/v (veterinary product) Each 5 ml of the suspension contains: 4. For control and treatment of 07.08.14 Gastrointestinal and pulmonary Levamisole HCl IP 112.5 mg eq to Nematodes, liver Flukes and levamisole 95.45 mg + Oxyclozanide IP Amphistomes. 150 mg + Anhydrous Copper Sulphate BP eq to elemental copper 1.25 mg + Zinc sulphate monohydrate USP eq to elemental Zinc 0.4 mg + Cobalt sulphate eq to elemental cobalt 0.15 mg (veterinary product) Each Film coated tablet contains: 5. the treatment of acute Piperaquine tetraphosphate as Piperaquine For uncomplicated P. Falciparum malaria Tetraphosphate Tetrahydrate (QP) 320 mg only for adult patients. + Dihydroartemisinin (DHA) 40 mg 18.09.14 Each 5 ml of reconstituted suspension contains: 6. Amoxicillin Trihydrate IP eq to Amoxicillin 200 mg/400 mg + Potassium Clavulanate IP Eq to Clavulanic acid 28.5 mg / 57 mg For the treatment of bacterial infection such as Sinusitis otitis media, Tonsilitis, acute and chronic bronchitis skin and soft tissue infections and post operative pain 18.09.14 For the treatment of patients with primary hypercholesterolemia. 23.09.14 For the treatment of bacterial infection such as Sinusitis otitis media, Tonsilitis, acute and chronic bronchitis skin and soft tissue infections and post operative pain 14.10.14 (additional Strength) Each Uncoated tablet contains: 7. Ezetimibe 10 mg + Simvastatin 40 mg (additional strength) Each combipack contains: A. Each 5 ml of constituted suspension contains: 1. Amoxicillin Trihydarte IP eq to Amoxicillin 200 mg + Potassium Clavulanate IP eq to Clavulanic acid 28.5 mg 8. 2. Amoxicillin Trihydarte IP eq to Amoxicillin 400 mg + Potassium Clavulanate IP eq to Clavulanic acid 57 mg B. One vial of purified water IP Each Vial contains: Purified water IP 30 ml (additional Pack) Each 1 ml of the constituted suspension contains: 9. Amoxicillin Trihydrate IP eq to Amoxicillin 80 mg+ Potassium Clavulanate IP eq to Clavulanic Acid 11.4 mg (additional Strength) For the treatment of infections due to susceptible isolates of the designated bacteria in the conditions such as lower Respiratory Tract Infections, Acute Bacterial Otitis Media, Sinusitis, Skin And Skin Stucture Infections, Urinary Tract Infections in Children more than 20 kg body weight. 14.10.14











