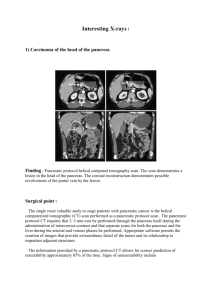
Supplementary Information (doc 62K)

Supplementary Materials and Methods
Pathological examination
All of the cases were pathologically examined and classified according to the World Health Organization (WHO) classification (Hruban et al , 2010), the International Union Against Cancer (UICC) tumor-node-metastasis
(TNM) classification (Sobin et al , 2009), and the Classification of Pancreatic Carcinoma of the Japan Pancreas
Society (Japan-Pancreas-Society, 2011). Surgically resected specimens were fixed in 10% formalin and the entire specimens were cut into serial slices 5 mm thick, horizontally in the pancreas head, and sagittally in the pancreas body and tail. All of these sections were then subjected to detailed pathological examination after staining with hematoxylin and eosin. For evaluation of veins and arteries, tissue sections were stained for elastic fibers.
All of the examinations of tissue specimens in this study, such as identification of TLOs, immunohistochemistry, and evaluation of cancer invasion to vessels or nerves, were done by using the maximum cut surface of the tumor.
In cases of chronic pancreatitis, we selected tissue slides containing typical feature of chronic pancreatitis that were at least 2 cm distant from the nearest cancer tissue. These were cases of pancreatic body cancer in which cancer-associated chronic obstructive pancreatitis had developed in the pancreatic tail.
Immunohistochemistry
We used 4-µm-thick sections of representative blocks with antibodies listed in Supplementary Table 2 . After immunohistochemistry (Supplementary Fig. 1B), quantitative evaluation of tumor-infiltrating immune cells was carried out as described previously (Ino et al , 2013b). Briefly, the microscopic images were imported as digital photo files using a NanoZoomer Digital Pathology (NDP) system (Hamamatsu Photonics, Japan), and we selected three areas at low magnification in which the immunolabeled cells had infiltrated into the tumor most densely. We did not select areas into which infiltrated immune/inflammatory cells had been recruited as a result of secondary tumor effects, such as pancreatitis, necrosis, ulceration, or mucus flooding of the tissue. Using NDP View at a magnification of x200, immunolabeled lymphocytes, neutrophils, or macrophages were then counted by two independent investigators. Since anti-CD3, CD11c, and CD20 antibodies were clearly reactive with the target
1
immune cells without any background, tumor-infiltrating CD3 + T cells, CD11c + DCs, and CD20 + B cells were evaluated quantitatively as follows: immune-labeled tumor slides were scanned with a NDP system, we measured the area of immune-labeled cells within the PDC tissues (mm 2 ) and the area of the PDC tissue (mm 2 ) using Image
J software (NIH), and then calculated the ratio of the two. The total area of S-100 protein (S-100)-positive nerve fibers morphologically selected within PDC tissue limited to within pancreatic tissue was quantified in the same way and compared with the total area of PDC tissue limited to within the pancreatic tissue. For triple-labeling of antigens, the sections were deparaffinized through a graded ethanol and xylene series, treated with 0.3% hydrogen peroxide in methanol for 30 min, and then autoclaved in 10 mM citrate buffer (pH 6.0) for 10 min at 121˚C. The initial immunohistochemical detection of alpha-smooth muscle actin was done by the avidin-biotin-peroxidase complex method with visualization using diaminobenzidine tetrahydrochloride solution. The antibodies were detached by acid treatment; the sections were incubated in 100 mM glycine/HCl (pH 2.2) for 15 min at room temperature with gentle stirring twice. The second immunohistochemistry for ERG was performed by the avidin-biotin-peroxidase complex method followed by visualization with VIP (Vector Laboratories). The antibodies were detached by acid treatment followed by counterstaining with hematoxylin. The third immunohistochemistry for podoplanin was performed by the avidin-biotin-peroxidase complex method followed by visualization with TMB (Vector Laboratories).
Double immunofluorescence
We performed double-immunofluorescence staining on formalin-fixed paraffin-embedded sections as described previously (Hiraoka et al , 2006). After immunofluorescence, the slides were scanned with a NanoZoomer Digital
Pathology system. We randomly selected three areas [average 0.80 (0.18-2.17) mm 2 ] in PDC tissue, measured the total immunofluorescence density of VE-cadherin in ERG + endothelial cells in the area, and calculated the average of their three data as the final value. When we performed double-immunofluorescence staining on
100µm-thick sections, immunostained tissue sections were analyzed with a confocal microscope (LSM5 Pascal;
Carl Zeiss Jena GmbH, Jena, Germany) equipped with a 15-mW Kr/Ar laser.
2
Extraction of RNA and real-time RT-PCR
Total RNA was extracted from fresh frozen samples of both tumor tissue and non-cancerous pancreas tissue, as described previously (Hiraoka et al , 2011). Non-cancerous pancreas tissues were obtained from the pancreatic tissue at least 2cm distant from the pancreatic cancer.
All samples were treated with rDNase during isolation, in accordance with the manufacturer’s instructions. The quality of the extracted RNA was measured using an
Agilent 2100 Bioanalyzer with a RNA 6000 Nano kit (Agilent Technologies, Santa Clara, CA). We measured and calculated the quality of total RNA extracted from tissues of 26 randomly selected PDCs (Supplementary Table 3).
The rRNA ratio [28s/18s] and RNA integrity number (RIN) were 1.21±0.18 and 7.2±0.9 (average ± SD), respectively.
Quantitative RT-PCR for target genes and non-target housekeeping control genes was performed with a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA) using FastStart Universal Probe
Master (ROX) and probes from the Universal Probe Library (Roche Diagnostics Corp., Indianapolis, IN), as described previously (Ino et al , 2013a). The sequences of the primers and the respective Universal Probe Library probes are given in Supplementary Table 4 . The CT values of the genes were mentioned in Supplementary Table
5 and the normalization of the expression levels of genes was done by expression of ACTB gene.
Evaluation of cancer invasion to arterioles and venules
We detected arteriole and venule pairs in tissue sections stained for elastic fibers using light microscopy. The microscopy images were imported as digital photo files using a NanoZoomer Digital Pathology system. We counted the numbers of arteriole and venule pairs within the PDC tissue, and also measured the area (mm 2 ) of the
PDC tissue using Image J software. We then calculated the density of arteriole and venule pairs in the area of
PDC.
Vascular invasion by cancer cells was detected in tissue sections stained for elastic fibers with hematoxylin and eosin using light microscopy (Supplementary Figs. 2A and 2B). When counting numbers of paired vessels, we assessed the numbers of arterioles or venules that cancer cells had invaded, and then calculated the frequency of
3
arterioles or venules that showed cancer invasion relative to the total number of arterioles or venules in PDC tissue.
Statistical analysis
Comparisons of variables were performed using Fisher’s exact test or chi-squared test based on their categorical data. Mann Whitney U-test was used to analyze differences between two groups with unequal variance. The postoperative proportion of overall survival (OS) and disease-free survival (DFS) was calculated by the
Kaplan-Meier method. Univariate analysis was performed for prognostic factors using the log-rank test. The factors found to be significant by univariate analysis were subjected to multivariate analysis using the Cox proportional hazards model (backward elimination method). Differences at P <0.05 were considered statistically significant. Statistical analyses were performed with StatView-J 5.0 software (Abacus Concepts, Berkeley, CA).
4
References
Hiraoka N, Onozato K, Kosuge T, Hirohashi S, Hiraoka N, Onozato K, Kosuge T, Hirohashi S (2006) Prevalence of FOXP3 + regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 12: 5423-34
Hiraoka N, Yamazaki-Itoh R, Ino Y, Mizuguchi Y, Yamada T, Hirohashi S, Kanai Y (2011) CXCL17 and ICAM2 are associated with a potential anti-tumor immune response in early intraepithelial stages of human pancreatic carcinogenesis. Gastroenterology 140: 310-21
Hruban RH, Boffetta P, Hiraoka N, Iacobuzio-Donahue C, Kato Y, Kern SE, Klimstra DS, Kloppel G, Maitra A,
Offerhaus GJA, Pitman MB (2010) Ductal adenocarcinoma of the pancreas. In World Health Organization
Classification of Tumours. Pathology & Genetics. Tumours of the Digestive System , Bosman FT, Carneiro F,
Hruban RH, Theise ND (eds), 4th edn, pp 281-91. Lyon: IARCPress
Ino Y, Yamazaki-Itoh R, Oguro S, Shimada K, Kosuge T, Zavada J, Kanai Y, Hiraoka N (2013a) Arginase II expressed in cancer-associated fibroblasts indicates tissue hypoxia and predicts poor outcome in patients with pancreatic cancer. PLoS One 8: e55146
Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N (2013b) Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer 108: 914-23
Japan-Pancreas-Society (2011) Classification of Pancreatic Cancer , 3rd English edn. Tokyo, Japan: Kanehara
Sobin LH, Gospodarowicz MK, Wittekind C (2009) UICC TNM Atlas , 7th edn. Hoboken, NJ: Wiley-Blackwell
5
6