CHEMISTRY COURSE OUTLINE-YEAR 12
advertisement

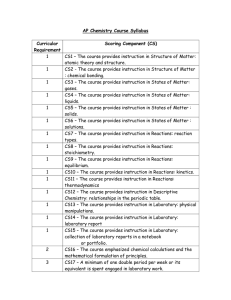
CHEMISTRY COURSE OUTLINE-YEAR 12 SEMESTER 1-2014 UNIT 3A CHE: CHEMICAL PROCESSES WEEK 1 WEEK 2 ORIENTATION a) course outline b) teaching/learning program c) assessment outline, assessment policy d)classroom rules/policies and procedures e) Review of past year syllabus content Atomic structure and Periodic Table structure of the atom in terms of protons, neutrons and electrons Shell electron configuration Ionisation Energy and Trends WEEK 6 -relationships between physical properties including melting and boiling point, and the types of intermolecular forces present. trends in melting and boiling points of hydrides of groups 15, 16 and 17 of NH3, H2O and HF interaction between solute and solvent particles in a solution ASSIGNMENT 1 ANNOUNCED atomic radius electronegativity Valence electrons The Periodic Table relationship between the number of valence electrons and an element’s bonding capacity position on Periodic Table physical and chemical properties WEEK 7 water’s ability to dissolve ionic, polar and non-polar solutes WEEK 3 Bonding Metallic bonding and Metallic Substances Ionic bonding and Ionic substances Naming Ionic Compounds WEEK 8 Revision for Atomic Structure Test. ASSIGNMENT 1 DUE TEST 1: Atomic Structure and Bonding. CHEMICAL REACTIONS Reactions, equations and stoichiometry Types of Chemical Reactions Review ASSIGNMENT 1 DUE Observations for the following reaction types: precipitation solvation of ions in aqueous solution ionic equations appropriate to the chosen context using ions. Kinetic Theory and Absolute zero calculations involving : - significant figures conversion between Celsius and Kelvin temperature scales WEEK 4 covalent network and covalent molecular substances chemical formulae for molecular compounds based on the number of atoms of each element present as inferred from the systematic names molecular formulae of commonly encountered molecules that have nonsystematic names Valence Shell Electron Pair Repulsion (VSEPR) theory and Lewis structure diagrams, shape of molecules and polyatomic ions (octet only) WEEK 5 Bonding polar and non-polar covalent bonds relationship between molecular shapes, bond polarity, and the polarity of molecules Bonding intermolecular and intramolecular forces dispersion forces dipole-dipole attractions hydrogen bonds ion-dipole interactions such as solvation of ions in aqueous solution WEEK 9 Final Concentration of Diluted and Mixed solutions WEEK 10 calculations involving : limiting reagent, and excess reagents mass, molar mass, number of moles of solute, concentration and volume of solution and gas volume using PV=nRT percentage purity of reactants or percentage yield in industrial processes TEST 2: CHEMICAL REACTIONS Equilibrium enthalpy (H), endothermic and exothermic reactions enthalpy diagrams WEEK 11 collision theory Factors Affecting Reaction Rates characteristics of a system in dynamic chemical and physical equilibrium equilibrium law expressions for homogeneous and heterogeneous systems WEEK 12 Using K values and equilibrium law expression to explain the relative proportions of products and reactants in a system in dynamic chemical equilibrium Use of Le Châtelier’s principle to predict the impact of changes in temperature changes in solution concentration changes in partial pressure of a gas addition of a catalyst. WEEK 16 to a system initially at chemical equilibrium: Applied chemistry equilibrium in biological, environmental or industrial processes WEEK 17 Final Exam Final Exam WEEK 13 variation of gas solubility in aqueous solution with temperature reasons for compromises between the ideal and actual conditions used in industrial processes that involve reversible reactions WEEK 14 WEEK 15 ASSIGNMENT 2 DUE investigate real world problems in a laboratory setting, considering: sources of uncertainty in experimental measurements selection of the appropriate units of measurement of quantities such as volume and time Revision for Exams. TEST 3: EQUILIBRIUM AND APPLIED CHEMISTRY ASSIGNMENT ANNOUNCED 2 WEEK 18 Exam Review Teaching and Exam Reviews Chemistry Art Project Closing Classroom Exercise WEEK 19 Assessment: Investigation Report due Revision for Final Exams