Lab 28 notes: Dilution Series Problems and
advertisement

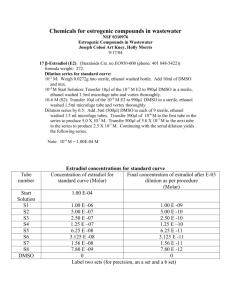
LAB 28 DILUTION SERIES PROBLEMS AND THE SPECTROPHOTOMETER For sample dilution problems, go to Google and type in “dilution problems” and click on “images”. Remember that OCD = colony count / (FDF)(PF). The colony count must be between 30-300 or it is not used. The FDF is from whichever tube that was used to pour the plate you are counting. Sample Dilution Quiz Suppose we have a water sample in which we want to measure the amount of bacteria. Transfer 1 ml into 99ml of sterile water. Transfer 10ml into 90 ml sterile water. Transfer 1 ml into 9ml sterile water. Plate 0.1 ml from each of the three tubes. The colony counts for plate A is 461, B is 45, and C is 1 What is OCD (Original Cell Density)? 1ml 10 ml 1ml Water Sample 99ml 90ml 0.1ml 461 9ml 0.1ml 45 0.1ml 1 Solution To determine OCD, we need to find the colony count, the plating factor, and the final dilution factor. First, we determine that plate B is the only countable plate. We therefore know the colony count is 45. The plating factor is 0.1 ml, but we should write this as 10-1. Now we have to find the final dilution factor. Use the formula: D = V1/V2 To find V1 for tube 2, we have to calculate all the previous dilutions up until we reach the countable plate. The first tube had 1 ml added to it, so that tube’s V1 = 1. The total volume for tube 1 is 99 + 1 = 100ml. Therefore, the first tube’s dilution factor is 1/100. This should be written as 10-2. The second tube had 10ml added to it, so its V1 = 10. The total volume on that tube is 90 + 10 = 100, so V2 = 100. Therefore, D = 10/100 for tube 2. That is the same as 1/10. That is the same as 10-1 To find the FINAL dilution factor, we multiply (10-2)(10-1). This gives us 10-3 (just add the exponents). Now we can apply the formula: OCD = colony count/(PF)(FDF) OCD = 45/(10-1)(10-3) OCD = 45/(10-4) Now remove the division sign and replace it with a multiplication sign by changing the exponent from negative to positive: OCD = 45 x 104 Now move the decimal point one place to the left by adding one to the exponent. OCD = 4.5 x 105 cfu/ml If you are not strong in math, repeat the above sample exercise at home five times. Then do it five more times, but substitute different values for the amount of sample added to each tube. For instance, in the first tube we added 1ml; change it to 10 ml. In the second tube we added 10ml; change it to either 1ml or 100ml. Remember to change the amounts for your sterile water in each tube as well so the total amount is either 100 or 10 or something else easy to work with. Now let’s try another dilution scheme using microliters (μl) instead of milliliters (ml). Sample problem We want to count the bacteria in a water sample. Transfer 10μl into 990 μl of sterile water in a tube. Then transfer 100μl from tube 1 to tube 2, which has 900μl sterile water. Both tubes have a plate factor of 100μl The colony count of plate A is TMTC. The colony count of plate B is 45. What is the OCD? Remember, OCD is in ml, not μl. 10μl 100μl Water Sample 990μl 900μl 100μl TMTC 100μl 45 Solution We know the colony count is 45. The plate factor is 100μl, which equals 0.1ml, which should be written 10-1 We must find the FDF by finding the dilution of tubes 1 and 2. V1 for tube 1 = 10μl V2 for tube 1 is 990 + 10 = 1000μl D for tube 1 is 10/1000. Eliminate the last zero from the numerator and the denominator so that D = 1/100. That is the same as D = 10-2. V1 for tube 2 is 100μl V2 for tube 2 is 900 + 100 = 1000μl D for tube 2 is 100/1000 Eliminate two zeros from the numerator and the denominator so that D = 1/10 That is the same as D = 10-1 Now multiply the dilution factors for tube 1 and tube 2 to get the FDF: (10-2)(10-1) = 10-3 Now apply the formula: OCD = 45/(10-1)(10-3) OCD = 45/10-4 OCD = 45 x 104 OCD = 4.5 x 105 μl Now we have to multiply by 1000 to convert μl to ml (just raise the exponent by 3). OCD = 4.5 x 108ml NOTE: you cannot have a dilution series that uses both μl and ml. The series has to be all in ml or μl but not both. Now let’s try a dilution series using milk instead of water. Sample problem: We want to measure the amount of bacteria in milk. Take the milk sample and add 0.1 ml to a tube of 99.9ml of sterile water. Transfer 1 ml to a tube of 99 ml sterile water. Transfer 1 ml into a tube of 9ml sterile water. From tube 2, make two plates: use 1ml for the first plate, and 0.1 ml for the second plate. From tube 3, make two plates: use 1 ml for the first plate, and 0.1 ml for the second plate. The plate counts turn out to be: Tube two: A = 525, B = 50 Tube three: C = 7, D = 0 What is the OCD? 1ml 1ml 0.1ml Milk Sample 99ml 99.9ml 1ml 525 9ml 0.1ml 50 0.1ml 1ml 7 0 Solution The only countable plate is plate B, which was from tube 2, the plate factor for which was 0.1ml, which is 10-1. To get the FDF, we have to find the dilutions on tube one and tube two, and then multiply them. V1 for tube one = 0.1 V2 for tube one = 99.9 +0.1 = 100 D for tube one = 0.1/100 To get rid of the decimal point, add a zero to the numerator and the denominator: D = 1/1000; this should be written as 10-3 V1 for tube two = 1 V2 for tube two = 99 + 1 = 100 D for tube two = 1/100 This should be written as 10-2 To get FDF, multiply the dilution factor for tubes one and two: FDF = (10-3)(10-2) FDF = (10-5) Now apply the formula: OCD = 50/(10-1)(10-5) OCD = 50/10-6 OCD = 50 x 106 OCD = 5.0 x 107 cfu/ml Now let’s try a problem where the sample is a solid instead of a liquid. With a solid, substitute grams (g) for ml and express the final answer as cfu/g Solids are put in a blender with some sterile water to make a slurry out of them to use in a dilution series. Sample problem Suppose we want to find out how many bacteria are in some raw hamburger. Take 1 gram of the hamburger slurry, and add it to 99 ml of sterile water. Transfer 0.1 ml to 99.9 ml sterile water. Transfer 1 ml to 9ml of sterile water. From the third tube, make two identical plates: use 1 ml for both plates. This is called a plate duplicate. In cases like these, add the colony counts together and divide by 2 to get the average for both plates and use that figure as your colony count. What is the OCD? Are you going to eat that meat? 1g 0.1ml 1ml Hamburger Sample 99ml 99.9ml 1ml 9ml 201 Solution The colony count average is 201 + 210 = 411 411/2 = 205.5 (Round that up to 206) The plate factor is 1 V1 for tube one = 1 V2 for tube one =99+1 = 100 D for tube one = 1/100 D for tube one = 10-2 V1 for tube two = 0.1 V2 for tube two = 99.9 + 0.1 = 100 D for tube two = 0.1/100 D = 1/1000 D = 10-3 V1 for tube three = 1 V2 for tube three = 9 + 1 = 10 D for tube three = 1/10 D = 10-1 Now multiply all three dilution factors: (10-2)(10-3)(10-1) = 10-6 Now apply the formula OCD = 206/(1)(10-6) OCD = 206/10-6 OCD = 206 x 106 OCD = 2.06 x 108 (Now round to the nearest tenth) OCD = 2.1 x 108 cfu/g No, I would not eat that meat! 1ml 210 HOW TO MAKE YOUR OWN DILUTION SCHEME The E. coli sample we started with was a 24 hour culture, so we would expect it to have between 5 x 107 and 5 x 10 9 cfu/ml. Sample problem Devise a dilution scheme to give us countable plates. Solution First, select the middle number in the range above (5 x 108) as our target OCD. What do you have to do to get a countable plate with this number (30-300)? Select a plate count that is a multiple of 10 to make the math easy. Let’s try a colony count of 50. We want to make 5 x 108 into 50. What do we have to do to make “5” into “50? Multiply by 10. That means we have to lower the exponent by one. Therefore: 5 x 108/107 = 50. The math checks out correctly, but remember, the dilution factor has to have a negative exponent, so the DF is actually 10-7. From our batch of E coli, transfer 10μl into a tube of 990 sterile water. What is our dilution so far? 10/1000 = 1/100 = 10-2; not dilute enough (needs to be 10-7), so keep diluting. Transfer 10μl from tube one into tube two with 990 μl sterile water? What is our dilution now? (10-2)(10-2) = 10-4; still not enough. Keep diluting. Transfer 10μl from tube two into tube three with 990 μl sterile water. What is the dilution now? (10-2) (10-4) = 10-6. This is still not enough, but the next dilution will be too much. Therefore, we stop now and dilute a little with the plating factor. Plate out 100μ from tube three. OCD = 50/(10-1)(10-6) OCD = 50/10-7 OCD = 50 x 107 OCD = 5.0 x 108 cfu/ml Tougher problem Start with one gram of Fido Dog Food and add 99 ml of sterile water. Blend into a slurry. Do four successive 1 ml dilutions into 9ml of sterile water. On the last dilution, remove 2ml and add to 8ml of buffer. Plate out 0.2 ml from this dilution. The plate will wind up with 200 colonies. What is OCD? 2ml 1g 1ml 1ml 1ml 1ml Fido Dog Food 99ml 9ml 9ml 9ml 9ml 8ml 0.2ml 200 Solution D1 = 10-2 D2 = 10-3 D3 = 10-4 D4 = 10-5 D5 = 10-6 D for tube 6 = (2 x 10-1)(10-6) = 2 x 10-7 PF = 0.2 = 2 x 10-1 OCD = 200 / (2 x 10-1)(2 x 10-7) OCD = 200 / 4 x 10-8 OCD = 200 / 4 (same as “50”) x 108 OCD = 50 x 108 OCD = 5.0 x 109 cfu/g From the last lab period, count your plates and record your data. Also record your colony counts for each plate and each group on the board: GROUP 1 GROUP 2 GROUP 3 GROUP 4 A B C D OCD To count your colonies, use the electronic colony counting device and the colony counter magnifying light. Set the counter to zero. Place your plate under the magnifier. Don’t bother with TFTC or TMTC plates. Touch each colony. An ink mark will be left on top of each colony. Make sure each colony is marked. Record your data and calculate the OCD. REASONS FOR ERRORS IN RESULTS Usually errors are from incorrect use of the pipettes: Plunger depressed all the way to suck up sample instead of to first position. Last drop in tube is not wiped off the pipette. Sucked up an air bubble After the class writes their results on the board, take the average OCD and see who guessed the closest from last lab and gets a candy bar! For your Data Sheets, fill out the info for the best plate and put in the class average that was found. SPECTROPHOTOMETER This is another method that is used to count bacteria. Within this machine, light comes out from a source and goes through a dispersion devise (a prism), which adjusts the wavelength by spreading all the light out into the full spectrum of the rainbow colors. This spread-out light then passes through the sample liquid in a special test tube called a cuvette, which is high quality glass with no imperfections that would distort the light. Whatever light can pass through the sample is detected on the other side of the machine. Cells or other causes of turbidity will refract the rest of the light away so it does not get detected. You can adjust the prism in the machine so it will only put out a red wavelength light. This is useful in measuring the amount of green chlorophyll in an algae sample. A spectrophotometer measures the turbidity and absorbance in percent transmission (%T). It also measures the optical density (OD). The spectrum of colors ranges: 700nm (red) – orange – yellow – green – blue – indigo – violet (350nm) Since you can set the prism to measure certain wavelengths, we can measure DNA at 260 nm to determine the concentration of DNA in a sample. The spectrophotometer measures both absorbance and turbidity, but we only want to measure the turbidity in our E. coli sample. How do we eliminate absorbance? We will use sterile nutrient broth as the diluent, and then pick a wavelength which is the same amber color as NB. The proper wavelength for this amber is 686nm. Set up a dilution scheme (standard curve for the spectrophotometer). Start with the batch of E. coli. Transfer 2ml into a tube of 2ml sterile water. Transfer 2ml into another tube of 2ml sterile water. Transfer another 2ml into a third tube of 2ml sterile water. Transfer another 2ml into a fourth tube of 2ml sterile water. The dilution of tube 1 = ½ The dilution of tube 2 = ¼ The dilution of tube 3 = 1/8 The dilution of tube 4 = 1/16 Now make a table to fill out from the spectrophotometer readings: Dilution ½ 1/4 1/8 1/16 % Transmission Optical Density For test questions: Know the name of this instrument. Why is it used? To measure absorbance and transmission. Why measure absorbance? To find concentration of a colored substance such as chlorophyll. When measuring absorbance, use wavelength to differentiate from the color of your liquid. A special unit called an ultraviolet spectrophotometer can measure proteins and nucleic acids. DNA is measured at 260 nm Proteins are measured at 270 nm. Our spectrophotometer just uses the visible spectrum of light. How do you change the wavelength? Adjust the prism. Dial in 686 (color of NB) to eliminate absorbance. Now it will only measure turbidity (cloudiness). SPECROPHOTOMETER GRAPH Use the graph paper on page 557 of your lab manual. Plot the dilution of the E. coli culture from the spectrophotometer readings on the x-axis. Plot the optical density on the y-axis. On the x-axis, plot the Dilutions 40 squares to the right, mark the graph for ½ dilution. On the 20th square to the right, mark for ¼ dilution. On the 10th square, mark the 1/8 dilution On the 5th square, mark the 1/18 dilution On the y-axis, plot the Optical Density 10 squares up = 0.1 20 squares up = 0.2 30 squares up = 0.3 Now plot the coordinates as the spectrophotometer indicated: ½ = 0.29 ¼ = 0.15 1/8 = 0.1 1/16 = 0.03 Draw a straight line that fits the points best. Note that one point falls outside of the straight line. This can be due to error in pipetting or the cuvette was dirty. QUESTIONS 1. What does the resulting standard curve indicate? The OD is directly proportional to cell density and cell density is directly proportional to dilution. Direct proportionality means that a ½ dilution has twice as many cells as a ¼ dilution. Problem If the OCD was 4.4 x 108, what is the cell density (cfu/ml) of the ½ dilution? Solution ½ of 4.4 x 108 is 2.2 x 108 Now we see a correlation (relationship) between optical density and cell density. 2. How can you use OD data to correlate with the cell density? Use a ratio proportion equations to solve for x. Problem Since we know from our graph that 2.2 x 108 has an optical density of 0.3, what is the cell density of an unknown sample of E. coli with an optical density of 0.2? Solution 0.2 0.3 = x___ 3.3 x 108 0.3x = (0.2)(3.3 x 108) x = (0.2)(3.3 x 108)/ 0.3 x = 2.2 x 108 cfu/ml Note that we got this answer without having to do a Standard Plate Count again. That means we can use the OD data to find the cell density on any E. coli sample by only doing one SPC. 3. When does the OD data NOT correlate with the cell density? The spectrophotometer will count dead cells as well as live ones. Therefore, in an old colony, the SPC numbers will decrease (dead cells don’t grow colonies), but the spectrophotometer will show and increase. That means you can only use the OD data on young colonies (16-18 hours old).