Bloodborne Pathogen Exposure and Gloves
advertisement

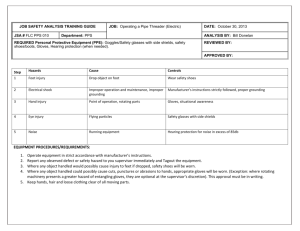
A Campus Safety Newsletter for Oklahoma’s Higher Education Institutions Coordinators’ Edition Gloves and Bloodborne Pathogens: All you ever wanted to know, but were afraid to ask. Latex. Vinyl. Nitrile. Powdered. Powder Free. Accelerator free. Class 1 Medical Device. NFPA 1999. ASTM F1671. ASTM F 739-96. FDA 21 CFR Part 800. AQL. ISO 9002. Examination Gloves. Latex Hypersensitivity. Low-Protein Latex. Hypoallergenic. NRL. Low Modulus. Type I dermatitis. Type IV Hypersensitivity. What does it all mean? William Stewart Halsted (1852-1922) was the first surgeon-in-chief of the new Johns Hopkins Hospital, established in 1889. He pioneered many surgical operations, as well as the use of rubber gloves for surgery. As quoted by Sherwin Nuland in Doctors: The Biography of Medicine, Halsted wrote the following description in a 1913 review article on surgical technique. "In the winter of 1889 and 1890 - I cannot recall the month - the nurse in charge of my operating room complained that the solutions of mercuric chloride produced a dermatitis of her arms and hands. As she was an unusually efficient woman, I gave the matter my consideration and one day in New York requested the Goodyear Rubber Company to make as an experiment two pair of thin rubber gloves with gauntlets. On trial these proved to be so satisfactory that additional gloves were ordered. In the autumn, on my return to town, an assistant who passed the instruments and threaded the needles was also provided with rubber gloves to wear at the operations. At first the operator wore them only when exploratory incisions into joints were made. After a time the assistants became so accustomed to working in gloves that they also wore them as operators and would remark that they seemed to be less expert with bare hands than with the gloved hands." In 1890 they only had one choice. Now, there are so many choices of gloves, how is anyone to know which glove is best to protect against bloodborne pathogens? The answer is not an easy one. Not all gloves are created equal. But first, some regulatory review. OSHA On December 6, 1991, the U.S. Occupational Safety and Health Administration (OSHA) enacted regulations requiring the use of work practice controls and protective clothing, including gloves, to minimize worker exposure to bloodborne pathogens (BBP). Since that time, glove use has skyrocketed and the issue of natural rubber latex (NRL) sensitivity has become a serious one. Latex sensitivity will be covered later in this newsletter. Medical Gloves: While OSHA does not directly state in 1910.1030 that gloves that are labeled “Class I medical device” are required to protect against BBP, an OSHA Technical Bulletin states: “The use of NRL as well as synthetic materials have been cleared for marketing as medical gloves (emphasis added) by the Food and Drug Administration (FDA) and can be used effectively for barrier protection against bloodborne pathogens.” And, “Since the reason for wearing gloves is to provide barrier protection from hazardous substances, substitute materials must maintain an adequate barrier protection and be appropriate for the hazard. At a minimum (emphasis added), gloves made from NRL or other materials and used for a medical purpose should be labeled as medical gloves.” Both OSHA statements can be found in the Technical Information Bulletin dated April 12: 1999; (http://www.osha.gov/dts/tib/tib_data/tib19990412.html). In another document (CPL 2-2.69), OSHA states: “Similar alternatives (to NRL) must supply appropriate barrier protection and must be approved by the FDA for use as a medical glove”. Although OSHA language does not directly state that medical gloves are required, it indirectly implies that all gloves used against BBP should be medical gloves as regulated by the FDA. Third Quarter 2003 Page 1 A Campus Safety Newsletter for Oklahoma’s Higher Education Institutions Coordinators’ Edition So, who should be wearing medical gloves? Health care workers, of course, but how about campus police? Employees who respond to minor first aid emergencies? Custodians who are trained to clean up spills of blood? Employers will have to make the final decision as to which employees are potentially exposed to human blood or other infectious materials and which employees should be wearing medical gloves during that exposure. Two final OSHA glove-related considerations: Regardless of which glove is selected, OSHA regulations are very clear that hands will be washed as soon as possible after glove removal, regardless of what kind of glove is used. The OSHA Enforcement Procedures (CPL 2-2.69) state that some petroleum-based hand creams can adversely affect glove integrity. Care should be taken when hand creams are used to avoid compromising the glove. CDC In 1987, the Centers for Disease Control and Prevention (CDC) recommended universal precautions when workers have contact with blood and certain body fluids. CDC has stated that the type of gloves selected should be appropriate for the task being performed. The following guidelines are recommended: 1. Use sterile gloves for procedures involving contact with normally sterile areas of the body (i.e., surgeon’s gloves). 2. Use examination gloves for procedures involving contact with mucous membranes, unless otherwise indicated, and for other patient care or diagnostic procedures that do not require the use of sterile gloves. FDA Because of the emphasis in the CDC recommendations upon gloves as a barrier to HIV, HBV and other blood-andfluid borne infectious agents, and the need for greater assurance against transmission between patients and health care workers, the Food and Drug Administration (FDA) has taken the lead to ensure that gloves worn by health care workers must provide an effective barrier to the transmission of infectious agents. Obviously, this effective barrier can be provided by ensuring that medical gloves meet appropriate standards and prevailing guidelines. The term “medical gloves” as used by FDA includes both surgeon’s gloves (sterile) and patient examination gloves (nonsterile). The test procedures established by the FDA are found at 21 CFR Part 800, Subpart B, Section 800.20(b). All medical gloves are tested using a water leak method. Based on an acceptable quality level (AQL), the FDA has determined a failure rate for surgeon’s gloves is 2.5 or higher, and 4.0 or higher for patient examination gloves. Manufacturers who wish to meet the FDA standard must complete an extensive application as well as undergo inspections. Manufactured products are sampled and then tested according to stringent FDA rules. In addition to the leak (pinhole) test, gloves are also tested for a variety of other characteristics such as tensile strength, thickness, and length. All manufacturers must also have design controls, documentation and process controls. Since 1987, the FDA has also: produced guidance to aid manufacturers in meeting FDA regulatory requirements and improving the quality of medical gloves; increased the sampling and testing of gloves; encouraged and supported the American Society for Testing and Materials (ASTM) in modifying existing standards and developing additional standards for medical gloves; and encouraged manufacturers to develop gloves with low levels of chemical residues and water-soluble proteins. Third Quarter 2003 Page 2 A Campus Safety Newsletter for Oklahoma’s Higher Education Institutions Coordinators’ Edition NFPA The National Fire Protection Association (NFPA) has written a testing standard for medical gloves that far exceeds that of the FDA. The standard was developed to address protective garments, gloves, and facewear designed to protect persons providing emergency medical care against exposure to liquid borne pathogens during medical emergency operations. This is NFPA Standard Number 1999. One of the unique features of NFPA 1999 is the ASTM F 1671 viral penetration test that measures penetration of the glove by a surrogate microbe under conditions of continuous contact. Other NFPA 1999 required tests include: a liquid integrity test (pinhole test) with an AQL of 1.5 (instead of 4.0) – ASTM D 5151 Strength (ultimate tensile strength, elongation, and modulus) – ASTM D 412 Ultimate Elongation (isopropyl degradation) – ASTM D 412 & ASTM D 3577 Puncture resistance – ASTM F 1342 Dexterity, and Protein levels (100 ug/g or less). Gloves that meet the NFPA standard are likely to be latex (but a few non-latex products are also available). They are also usually powder free, thicker than Class I medical exam gloves, have a longer cuff, and may also be called “high risk” or “EMS” gloves. Latex/Latex Allergies Natural rubber latex (NRL) is a milky fluid obtained in commercial quantities primarily from the Heavea brasiliensis tree. NRL contains variable amounts of proteins which may be absorbed through the skin or inhaled to cause an allergic reaction in susceptible persons. NRL protein absorption has been reported to be enhanced when perspiration collects under latex clothing articles. Lawsuits about latex sensitivity against suppliers, manufacturers and distributors are increasing. In one case, a radiologist won $1 million. Some hospitals have gone so far as to ban latex rubber balloons. Because of potential allergic reactions, many purchasers have eliminated latex gloves and are buying synthetics. According to Stephen Barrett, M.D., approximately 800,000 American adults and children have become allergic to NRL found in gloves and pacifiers. When exposed to latex or latex dust, sensitized persons can develop hives, nasal and eye irritation, asthma, and anaphylaxis, a life-threatening condition in which the breathing passageways swell closed. About 1% of the general public and 4.5% to 17% of health-care workers and others exposed to latex on their jobs have become sensitized. There are no OSHA standards specific to latex allergy and exposure, but OSHA has created a webpage devoted to the topic (http://www.osha.gov/SLTC/latexallergy/). However, 1910.1030(d)(3)(iii) states: “The employer shall ensure that appropriate personal protective equipment in the appropriate sizes is readily accessible at the worksite or is issued to employees. Hypoallergenic gloves, glove liners, powderless gloves, or other similar alternatives shall be readily accessible to those employees who are allergic to the gloves normally provided.” Hypoallergenic claim. Although the term “hypoallergenic” is found in the OSHA bloodborne pathogen regulation, FDA now prohibits the labeling of NRL Class I devices as "hypoallergenic." In addition, the FDA requires labeling statements on NRL medical devices, including NLR device packaging that contacts humans. The warning states: ``Caution: This Product Contains Natural Rubber Latex Which May Cause Allergic Reactions”. The Best glove company has published a three page report on gloves and allergies that can be found at: http://www.chemrest.com/what%20causes%20allergic%20reactions%20to%20gloves.pdf. Also, the OSHA Third Quarter 2003 Page 3 A Campus Safety Newsletter for Oklahoma’s Higher Education Institutions Coordinators’ Edition Technical Bulletin dated April 12, 1999 has detailed information on the different types of irritation and allergic reactions that can occur from NRL and other gloves. Type I allergic reactions can take place within seconds and may be life threatening, while Type IV reactions take longer. You can find this document at: http://www.osha.gov/dts/tib/tib_data/tib19990412.html. Glove Brands There are many brands of medical grade gloves available from manufacturers such as Ansell, Best, Microflex, Sensicare, and others. Depending on your specific requirements, look at their descriptions and call the manufacturers if you are not certain about the characteristics of the gloves you are considering. Considerations include but are not limited to: selection; size; glove materials; potential for chemical and/or infectious materials exposures and the severity of that exposure; and sensitivities of workers. Glove Terminology 101 Class I Medical Device: This is an FDA definition used for medical devices subject to only to general FDA controls. This device is not life-supporting or life-sustaining. (One glove manufacturer has recently told me that some Class I gloves may be changing to Class II gloves, but FDA regulations do not seem to shed any light on this statement at this time. Class II devices are subject to or will be subject to special controls, but it only means that controls will be even tighter if and when this change takes effect). Low Modulus: A low modulus glove is easy to stretch and flex, whereby a high modulus glove is hard to move and stretch. Modulus is really what most people think of as “elasticity”, or the “degree of stretch”. The higher the modulus, the more force is required to stretch the film. So “low modulus” gloves have a softer, more elastic feel and exert less pressure on the hands. Look for low modulus to minimize hand fatigue and chafing. (Note: some medical grade gloves are “medium modulus” depending on the brand). Powder/Powder free: Many people believe that they are allergic to glove powder. Most glove powder used to manufacture gloves is food-grade cornstarch and is not an allergy causing material. However, glove powder has been implicated in cases of latex allergy because it can serve a carrier of the natural rubber latex protein. The latex protein laden cornstarch can be inhaled. Accelerators: Gloves made of NRL and synthetics (with one exception) all contain chemical accelerators such as thiuram, carbamates, and benzothiazoles to which a worker may also develop sensitization, resulting allergic contact dermatitis. Antioxidants, biocides, soaps, and other chemicals used in processing NRL products may also contribute to sensitization. Only one glove, from Best, is currently marketed as accelerator free. Glove sizing: Select the size that gives you the right fit, dexterity and comfort. Oversized gloves can be hazardous while under sizing can cause fatigue. To determine your size, measure the hand circumference around the palm area, just beneath the knuckles. For example, 7" is equal to a size 7 glove. If the gloves you are ordering are sold in numbered sizes, choose the size that is closest to the number you get from tape measure. As an example, if your hand measures 9 3/4 inches, choose a size 10 if you want a loose fit or a 91/2 if you want a tight fit. If the gloves only come in whole sizes, choose the closest whole size. Follow specific manufacturer sizing guidelines, if any. Hand Sanitizers The new Centers for Disease Control and Prevention (CDC) "Guideline for Hand Hygiene in Health-Care Settings" (Morbidity and Mortality Weekly Report, October 25, 2002) supports the use of alcohol-based hand rubs as an effective means for decontaminating hands in healthcare settings. However, in an interpretation dated 3/23/2003, OSHA states: If a sink is not readily accessible (e.g., in the field) for instances where there has been occupational exposure, hands may be decontaminated with a hand cleanser or towelette, but must be washed with soap and running water as soon as feasible. If there has been no occupational exposure to blood or OPIM, antiseptic hand cleansers may be used as an appropriate "handwashing" practice. The full interpretation can be found at: http://www.osha.gov/SLTC/bloodbornepathogens/index.html under “new interpretation letters”. Third Quarter 2003 Page 4