Adiabatic and isothermal
advertisement
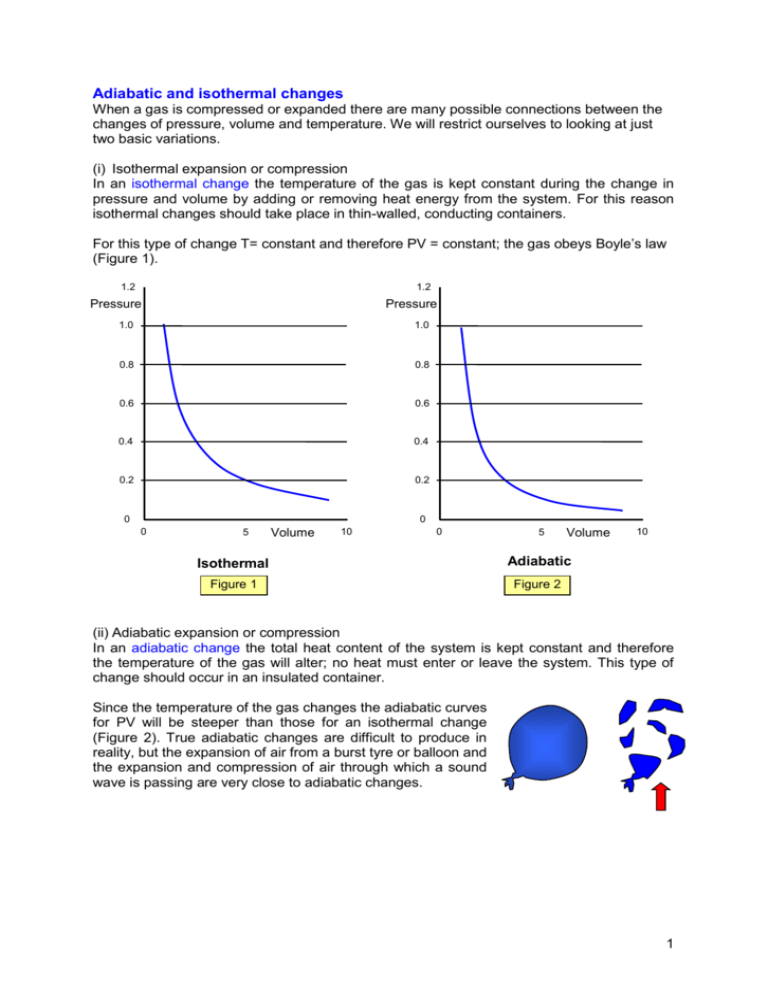
Adiabatic and isothermal changes When a gas is compressed or expanded there are many possible connections between the changes of pressure, volume and temperature. We will restrict ourselves to looking at just two basic variations. (i) Isothermal expansion or compression In an isothermal change the temperature of the gas is kept constant during the change in pressure and volume by adding or removing heat energy from the system. For this reason isothermal changes should take place in thin-walled, conducting containers. For this type of change T= constant and therefore PV = constant; the gas obeys Boyle’s law (Figure 1). 1.2 1.2 Pressure Pressure 1.0 1.0 0.8 0.8 0.6 0.6 0.4 0.4 0.2 0.2 0 0 0 5 Volume 10 0 5 Volume Isothermal Adiabatic Figure 1 Figure 2 10 (ii) Adiabatic expansion or compression In an adiabatic change the total heat content of the system is kept constant and therefore the temperature of the gas will alter; no heat must enter or leave the system. This type of change should occur in an insulated container. Since the temperature of the gas changes the adiabatic curves for PV will be steeper than those for an isothermal change (Figure 2). True adiabatic changes are difficult to produce in reality, but the expansion of air from a burst tyre or balloon and the expansion and compression of air through which a sound wave is passing are very close to adiabatic changes. 1 Isothermal graphs at different temperatures The following graphs show the PV curves for isothermal changes for a given mass at two different temperatures To and T1 where T1>To. 1.40 1.40 1.20 Pressure 1.00 1.20 Pressure 0.80 0.80 Figure 3 1.00 T1 To 0.60 0.60 0.40 0.40 0.20 0.20 0.00 0.00 0 2 4 6 Volume 8 10 0 2 4 6 Volume 8 10 Temperature variation in a reversible adiabatic change When a gas in an insulated container is compressed or expanded it suffers a change in temperature, the molecules of gas gaining energy from, or losing energy to, the moving walls of the container. Figure 4 (b) (a) (c) Consider a volume of gas enclosed in an insulating container by a frictionless piston. Let the initial velocity of a molecule moving in the x-direction be u. (a) If it collides with a stationary wall of the container its velocity after collision will be -u (see Figure 4 (a)). Now consider the case when the wall is moving at velocity v, first an expansion and then a compression (Figures 4(b) and (c)). (b) The velocity of the molecule relative to the wall = u - v. After collision: velocity relative to the wall = - (u - v) velocity relative to the Earth = - (u - 2v) The final velocity is less than the initial velocity by 2v, and so the gas has cooled. Notice that this cooling only takes place while the wall of the container is moving. (c) Similarly for the compression we can say that, after collision, the velocity relative to the Earth = - (u + 2v) This shows an increase in velocity, and therefore the temperature of the gas will be raised 2










