ROXITHROMYCIN - ERYTHROMYCIN COMPARISON
advertisement

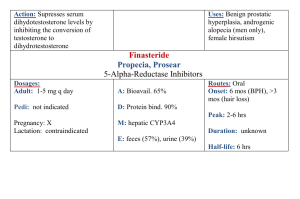
ROXITHROMYCIN - ERYTHROMYCIN COMPARISON PATIENT DATA/BACKGROUND: Please compare the pharmacokinetics, spectrum of activity, drug interactions and dosing, between roxithromycin and erythromycin. RESPONSE: Roxithromycin is an acid-stable macrolide antibacterial agent structurally related to erythromycin. Roxithromycin has antibacterial activity similar to that of erythromycin, but reportedly as superior pharmacokinetic properties. COMPARATIVE PHARMACOKINETICS Roxithromycin is more acid-stable than erythromycin, and achieves higher serum concentrations at equimolar oral doses. The half-life of roxithromycin is also longer than that of erythromycin (10 to 12 hours versus 1.5 to 3 hours) (Nilsen, 1987; Puri & Lassman, 1987; Young et al, 1989). Following oral administration roxithromycin 150 milligrams, mean peak plasma levels of 6.6 to 7.9 mcg/mL are achieved in 1 to 3 hours (Nilsen, 1987; Young et al, 1989) with doses of 300 milligrams orally, peak levels have ranged from 9.1 to 10.8 mcg/mL (Young et al, 1989). In comparison, peak serum levels following erythromycin stearate 500 milligrams orally have ranged from 0.5 to 2.9 mcg/mL, within 1 to 3 hours (Nilsen 1987; Kees et al, 1988). The area under the plasma concentration-time curve following roxithromycin 150 milligrams orally is approximately 8 to 10 times of that achieved following oral erythromycin base or stearate 500 milligrams, suggesting improved bioavailability and/or a lower clearance of roxithromycin. Tissue distribution of each drug is similar, although serum protein binding of erythromycin is lower than that of roxithromycin. Roxithromycin is bound extensively and saturably to alpha-1-acid-glycoprotein, and significantly increases free roxithromycin serum concentrations, which are observed when roxithromycin total serum concentrations exceed 4 mcg/mL (increase in free concentration from 4.3% to 13.4% when total serum levels rise from 3.3 to 8.4 mcg/mL). The binding of erythromycin to alpha-1-acid-glycoprotein exhibits a similar saturation phenomena, however the pharmacokinetic and clinical consequences of this binding are less than that observed roxithromycin, attributable to the lower degree of serum protein binding of erythromycin (Nilsen, 1987). Roxithromycin is not metabolized extensively, is excreted primarily unchanged, and does not interfere with hepatic microsomal enzyme systems (Periti & Mazzei, 1987; Delaforge et al, 1988; Puri & Lassman, 1987). Following roxithromycin 150 milligrams, 74% of the dose was accounted for, with fecal excretion, pulmonary excretion, and urinary excretion being 53%, 13%, and 7%, respectively (McLean et al, 1988). In contrast, erythromycin interacts with the cytochrome P-450 enzyme system, which can result in potential drug interactions (Young et al, 1989). A considerable portion of an oral dose of erythromycin is eliminated in the feces; 2% to 5% of an oral dose and 12% to 15% of an intravenous dose is eliminated in the urine (AMA, 1986). COMPARATIVE DOSING Based upon available pharmacokinetic data, roxithromycin 150 milligrams orally twice daily or 300 milligrams once daily should produce plasma concentrations above the minimum inhibitory concentrations required for antibacterial activity (Nilsen, 1987; Puri & Lassman, 1987). In infants and children, the recommended dose of roxithromycin is 2.5 to 5 milligrams/kilogram orally twice daily (Young et al, 1989). For erythromycin, the recommended dose in children is 30 to 50 milligrams/kilogram/day, in 4 divided doses (AMA, 1986). Some evidence exists suggesting that bacteriological cure rates are inadequate with once-daily dosing (Herron, 1987), and more clinical trials are required before a once-daily dosing regimen can be recommended. The recommended dose or erythromycin is 250 to 500 milligrams every 6 hours for the base, estolate, and stearate; doses of 400 to 800 milligrams 4 times daily are recommended for the ethyl succinate (AMA, 1986). However, lower doses of erythromycin have been employed clinically (ie, 500 milligrams every 12 hours), and comparative clinical trials are required to determine advantages of the superior pharmacokinetic profile of roxithromycin over erythromycin in the clinical setting. Dosing adjustments of roxithromycin are not required in the elderly or in patients with renal insufficiency (Puri & Lassman, 1987; Young et al, 1989; Nilsen, 1987). Although some investigators indicate that dosing adjustments are not required in patients with hepatic disease, including severe cirrhosis (Puri & Lassman, 1987; Periti & Mazzei, 1987), others suggest dose reductions to 150 milligrams orally once daily in patients with severe cirrhosis, due to the 2-fold increase in half-life observed in these patients (Young et al, 1989). Erythromycin may also be given without dosing adjustments in the elderly and in patients with renal impairment and alcoholic liver disease (Nilsen, 1987). However, some sources suggest that caution should be exercised in patients with impaired hepatic function, since erythromycin is excreted primarily by the liver (AMA, 1986). COMPARATIVE ANTIBACTERIAL ACTIVITY The antibacterial spectrum of roxithromycin is similar to that of erythromycin (Jones et al, 1983; Rolston et al, 1986; Young et al, 1989; Barlam & Neu, 1984). Both agents have similar minimum inhibitory concentration values for gram-positive organisms, including staphylococci, Streptococcus pneumoniae, Streptococcus pyogenes, and Bacillus cereus, and Corynerbacterium spp (Young et al, 1989; Pechere & Auckenthaler, 1987). Similar activity has also been observed between the 2 agents against Neisseria gonnorrhoeae, Neisseria meningitidis, and Branhamella catarrhalis (Young et al, 1989). Both agents are similarly active against anaerobic organisms, including Bacteroides spp (except Bacteroides fragilis) (Dubreuil, 1987; Young et al, 1989). Isolates of Chlamydia and Mycoplasma are also susceptible to roxithromycin, with minimum inhibitory concentration values (MIC-90) being similar to that observed with erythromycin (Young et al, 1989). Both erythromycin and roxithromycin are relatively inactive against the Enterobacteriaceae (Young et al, 1989; Jones et al, 1983). Legionella pneumophila is susceptible to both erythromycin and roxithromycin (MIC-90, 1 microgram/milliliter) (Young et al, 1989). Some studies suggest superior in vitro activity of roxithromycin over erythromycin against Legionella pneumophila (Hara et al, 1987), however, this is of doubtful clinical relevance. Serum concentrations of roxithromycin of less than 2 micrograms/milliliters inhibit most susceptible organisms, and these concentrations are easily maintained following doses of 150 milligrams orally every 12 hours (serum levels of 2.5 mcg/mL observed after 12 hours with this schedule). Most bacterial strains resistant to erythromycin are also resistant to roxithromycin (Young et al, 1989). COMPARATIVE EFFICACY In limited clinical trials, roxithromycin has been as effective as erythromycin in the treatment of upper and lower respiratory tract infections (Bertrand et al, 1988; Gentry, 1987; Herron, 1987; Melcher, (1987). Overall, roxithromycin 150 milligrams orally twice daily for 10 days has been effective in treating lower respiratory tract infections, eyes, nose, and throat infections, genitourinary infections, and skin and soft tissue infections, producing clinical cures in 83% to 100% of patients. Clinical cure rates of 92% to 100% have been observed in pediatric patients with a variety of infections employing roxithromycin 2.5 to 5 milligrams/kilogram every 12 hours (Young et al, 1989). In 1 single-blind study involving 227 patients, roxithromycin 150 milligrams orally twice daily was comparable to erythromycin ethyl succinate 400 milligrams orally 4 times daily in treating streptococcal throat infections (Herron, 1987). A satisfactory clinical response was observed in 83% and 88% of patients, respectively. Bacteriological cure was seen in 88% and 92% of patients treated with roxithromycin and erythromycin, respectively. In this study, roxithromycin 300 milligrams once daily was also administered in randomized fashion, resulting in a satisfactory clinical response in 87% of patients treated; however, the bacteriological response rate was 84%, which was significantly less than that with erythromycin. COMPARATIVE TOXICITY As with erythromycin, the most common adverse effects observed with roxithromycin include nausea, vomiting, abdominal pain, and diarrhea, each occurring in approximately 1% of patients treated (Blanc et al, 1987). The comparative incidence of adverse effects with erythromycin and roxithromycin is difficult to ascertain with available data. However, collective results of several studies have indicated a similar incidence of adverse effects, ranging from 2% to 14% with roxithromycin and 3% to 15% with erythromycin ethyl succinate (Gentry, 1987). In 1 single-blind study involving 227 patients with throat infections, the incidence of adverse reactions (primarily gastrointestinal in nature) was less with roxithromycin 150 milligrams orally twice daily as compared to erythromycin ethyl succinate 400 milligrams orally 4 times daily (Herron, 1987). Abnormalities in hepatic function tests have been observed in less than 1% of patients treated (Blanc et al, 1987). No cases of hepatitis have been observed to date with roxithromycin. Lymphopenia and eosinophilia have been observed rarely (Young et al, 1989). DRUG INTERACTIONS Due to the apparent lack of effect of roxithromycin on the cytochrome P-450 system, this agent is less likely than erythromycin to produce drug interactions mediated via this enzyme system (Delaforge et al, 1988). In available studies, roxithromycin has produced only minimal effects on the pharmacokinetics of theophylline (Saint-Salvi et al, 1987). In contrast, erythromycin increased the half-life of theophylline by 10% to 20% in most patients, as well as reducing theophylline oral clearance by 20% to 50% (Ludden, 1985). Roxithromycin also does not appear to affect the pharmacokinetics of carbamazepine (Saint-Salvi et al, 1987). Dosing adjustments of theophylline or carbamazepine do not appear to be required when given in combination with roxithromycin (Surjus et al, 1985; Saint-Salvi et al, 1987). Other reports suggest that roxithromycin does not interact with warfarin, ranitidine, or antacids (Young et al, 1989). CONCLUSION: Roxithromycin is an acid-stable macrolide antibacterial agent chemically related to erythromycin. The main advantages of roxithromycin over erythromycin include a potentially superior pharmacokinetic profile (higher serum concentrations, longer elimination half-life) and a lower potential for drug interactions. However, further clinical trials are required to compare these 2 agents and determine if the pharmacokinetic differences between these 2 agents are clinically relevant. Although a lower incidence of adverse reactions has been described with roxithromycin as compared to erythromycin in 1 clinical trial, more studies are required to determine if roxithromycin offers any advantage with regard to adverse effects. REFERENCES: 1. AMA Department of Drugs: AMA Drug Evaluations, 6th Ed, American Medical Association, Chicago, IL, 1986. 2. Barlam T & Neu HC: In vitro comparison of the activity of RU28965, a new macrolide, with that of erythromycin against aerobic and anaerobic bacteria. Antimicrob Agents Chemother 1984; 25:529-531. 3. Bertrand A, Caubarrere I, Chapman A et al: Multicentre comparative study of the efficacy and safety of roxithromycin and erythromycin ethyl succinate in the treatment of lower respiratory tract infections. Br J Clin Pract 1988; 42:98-99. 4. Blanc F, D'Enfant J, Fiessinger S et al: An evaluation of tolerance of roxithromycin in adults. J Antimicrob Chemother 1987; 20:179183. 5. Delaforge M, Sartori E & Mansuy D: Effects of roxithromycin on rat hepatic P-450 cytochromes: comparison with troleandomycin and erythromycin. Br J Clin Pract 1988; 42:67-69. 6. Dubreuil L: In-vitro comparison of roxithromycin and erythromycin against 900 anaerobic bacterial strains. J Antimicrob Chemother 1987; 20:13-19. 7. Gentry LO: Roxithromycin, a new macrolide antibiotic, in the treatment of infections in the lower respiratory tract: an overview. J Antimicrob Chemother 1989; 20:145-152. 8. Hara K, Suyama N, Yamaguchi K et al: Activity of macrolides against organisms responsible for respiratory infection with emphasis on mycoplasma and Legionella. J Antimicrob Chemother 1987; 20:75-80. 9. Herron JM: Roxithromycin in the therapy of Streptococcus pyogenes throat infections. J Antimicrob Chemother 1987; 20:139144. 10. Jones RN, Barry AL & Thornsberry C: In vitro evaluation of three new macrolide antimicrobial agents, RU 28965, RU 29065, and RU 29702, and comparisons with other orally administered drugs. Antimicrob Agents Chemother 1983; 24:209-215. 11. Ludden TM: Pharmacokinetic interactions of the macrolide antibiotics. Clin Pharmacokinetics 1985; 10:63-79. 12. McLean A, Sutton JA, Salmon J et al: Roxithromycin: pharmacokinetic and metabolism study in humans. Br J Clin Pract 1988; 42:52-53. 13. Melcher GP, Winn RE & Hadfield TL: Comparative efficacy, toxicity and compliance of RU 965 versus erythromycin ethylsuccinate (EES0 for streptococcal pharyngitis (SP). ASM annual meeting, Atlanta, March 1-6, 1987. 14. Nilsen OG: Comparative pharmacokinetics of macrolides. J Antimicrob Chemother 1987; 20:81-88. 15. Pechere JC & Auckenthaler R: In-vitro activity of roxithromycin against respiratory and skin pathogens. J Antimicrob Chemother 1987; 20:1-5. 16. Periti P & Mazzei T: Pharmacokinetics of rotithromycin in renal and hepatic failure and drug interacitons. J Antimicrob Chemother 1987; 20:107-112. 17. Puri SK & Lassman HB: Roxithromycin: a pharmacokinetic review of a macrolide. J Antimicrob Chemother 1987; 20:89-100. 18. Rolston KV, LeBlanc B & Ho PH: In vitro activity of RU28965, a new macrolide, compared to that of erythromycin. J Antimicrob Chemother 1986; 17:161-163. 19. Saint-Salvi B Tremblay D, Surjus A et al: A study of the interaction of roxithromycin with theophylline and carbamazepine. J Antimicrob Chemother 1987; 20:121-129. 20. Surjus A, Tremblay D, Saint-Salvi B et al: Pharmacokinetic interaction of a new macrolide, roxithromycin (RU28965) with theophylline. IIIrd World Conference on Clinical Pharmacology and Therapeutics, Stockholm, 1985; Abstract 1203. 21. Young RA, Gonzalez JP & Sorkin EM: Roxithromycin: a review of its antibacterial activity, pharmacokinetic properties and clinical efficacy. Drugs 1989; 37:8-41.

![njc34_4[^]](http://s3.studylib.net/store/data/007315276_1-991b477231a5bba08dd276919b6b9bbf-300x300.png)





