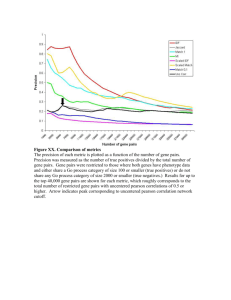
Supplementary A. B. C. Supplementary Figure 1. Morphology of
advertisement

Supplementary A. B. C. Supplementary Figure 1. Morphology of EML cells transduced with MSCV vectors. (A) Undifferentiated cells in normal culture medium. EML, EML-GFP, and EML-HOXB4 cells all had a similar morphology, with large vacuoles and the presence of cells having a hand mirror shape in regular growth medium. (B) Cells differentiated to the EPRO stage, maintained in culture medium with GM-CSF. Over 90% of the cells of each category had numerous azurophilic granules and kidney bean-shaped nuclei. (C) Cells differentiated to the neutrophil stage of maturation after culture in the presence of 10uM ATRA for 96 hrs. Typical ring-shaped and segmented nuclei are observed. Wright-Giemsa stain at the 45X magnification. Lineage Markers EPRO-EML EPRO-GFP EPRO-HOXB4 Stem/Progenitor Markers EPRO-EML EPRO-GFP EPRO-HOXB4 Supplementary Figure 2. Cell surface immuno-phenotype of cells differentiated to the EPRO stage of granulocytic maturation. By cell surface immuno-phenotyping, EPRO-EML, EPRO-GFP and EPRO-HOXB4 cells all had characteristics of granulocytic cells with similar patterns of expression of Gr-1 and Mac-1 in a high percentage of the cells. The percentage of cells expressing Sca-1, B220, Sca-1 and CD34 levels is markedly decreased in all three cell types when compared to EML cells. Supplementary Figure 3. Southern Blot analysis of DNA from single-cell clones of EML-GFP and EML-HOXB4 cells. Southern Blot analysis of DNA extracted from cultures of single-cell clones, digested with EcoRI and hybridized to a GFP probe. This analysis will reveal a single junction fragment for each integration site. Each single-cell clone has a different pattern of retroviral insertion sites. There are one to three integration sites per clone. This analysis helped to exclude the possibility that any of the phenotypes we observed in the EML-HOXB4 cells was due to a particular position effect of retroviral insertion and also provided us with true biological triplicates for use in our microarray studies. Supplementary Table 1. RNA expression profiling results. RNA expression profiling results comparing KLS-EML-B4 cells to KLS-EML-GFP cells. 465 gene transcripts were observed to be differentially expressed by at least 2-fold. PLEASE SEE ATTACED EXCEL SHEET Supplementary Table 2. ChIP-chip Results. ChIP-chip results using Tilescope software revealed that the promoter regions of 1910 genes were bound by HOXB4 PLEASE SEE ATTACED EXCEL SHEET Supplementary Table 3. This table lists the 71 differentially expressed genes, identified by microarray analysis in our RNA expression profiling experiments, that were also observed by ChIP-chip to have DNA binding sites occupied by HOXB4 in their proximal promoter region. The majority of these target genes (54/71) expressed transcripts that were up-regulated in KLS-EML-HOXB4 cells. The gene transcripts are ordered according to their rank in the ChIP-chip data set. Fold Difference HOXB4/GFP Gene Symbol 8.39 2.50 -2.53 3.65 Gp49a Rab38 2010301N04Rik Clec4e 9.82 2.76 2.19 2.13 Laptm4b AI586015 Arhgap15 Bmp2k -2.14 Rpl3 4.26 Kazald1 3.90 2.68 2.46 2.45 -2.73 Chchd6 Zfp521 Tm2d2 Fcgr2b Rad23a 2.17 2.79 2.07 2.45 2.64 Als2 Mfge8 Scap2 Rab19 1500041B16Rik -2.74 2.52 Orc5l Stat3 Gene Title glycoprotein 49 A /// leukocyte immunoglobulin-like receptor, subfamily B, member 4 Rab38, member of RAS oncogene family RIKEN cDNA 2010301N04 gene C-type lectin domain family 4, member e lysosomal-associated protein transmembrane 4B expressed sequence AI586015 Rho GTPase activating protein 15 BMP2 inducible kinase ribosomal protein L3 /// similar to 60S ribosomal protein L3 (J1 protein) Kazal-type serine peptidase inhibitor domain 1 coiled-coil-helix-coiled-coil-helix domain containing 6 zinc finger protein 521 TM2 domain containing 2 Fc receptor, IgG, low affinity IIb RAD23a homolog (S. cerevisiae) amyotrophic lateral sclerosis 2 (juvenile) homolog (human) milk fat globule-EGF factor 8 protein src family associated phosphoprotein 2 RAB19, member RAS oncogene family RIKEN cDNA 1500041B16 gene origin recognition complex, subunit 5-like (S. cerevisiae) signal transducer and activator of ChIPChip Rank (out of 1910) RNA Expression Profiling Rank (out of 465) 29 68 88 95 4 169 369 38 96 122 141 162 2 111 257 284 188 331 208 24 328 379 382 399 440 32 125 184 187 381 454 488 510 533 586 272 106 309 185 133 630 654 383 163 3.41 -2.38 2.08 2.50 4.42 2.90 3.25 2.53 Cd69 Clcn3 Il6 Zfp238 Cd34 Grcc10 Arhgap28 AI504432 2.93 Sox4 2.26 2.57 3.45 -4.52 3.83 Phtf2 Prkd2 Gpr171 Tsc22d1 Myct1 2.74 4.49 Ift57 Meis1 -3.21 2.61 Hmbs 4732435N03Rik 2.59 -2.36 2.26 Lat2 Fbxl19 Kifap3 2.04 3.47 -3.31 2.22 P2rx3 Lztfl1 Ifngr1 Gstm1 2.58 2.79 2.58 2.28 3.10 2.68 2.17 Slc39a6 Glipr1 Rasa2 Lrmp Cpne3 Msrb2 Hes6 transcription 3 CD69 antigen chloride channel 3 interleukin 6 zinc finger protein 238 CD34 antigen gene rich cluster, C10 gene Rho GTPase activating protein 28 expressed sequence AI504432 SRY-box containing gene 4 /// similar to Transcription factor SOX-4 putative homeodomain transcription factor 2 protein kinase D2 G protein-coupled receptor 171 TSC22 domain family, member 1 myc target 1 intraflagellar transport 57 homolog (Chlamydomonas) myeloid ecotropic viral integration site 1 hydroxymethylbilane synthase /// similar to hydroxymethylbilane synthase /// similar to hydroxymethylbilane synthase RIKEN cDNA 4732435N03 gene linker for activation of T cells family, member 2 F-box and leucine-rich repeat protein 19 kinesin-associated protein 3 purinergic receptor P2X, ligand-gated ion channel, 3 leucine zipper transcription factor-like 1 interferon gamma receptor 1 glutathione S-transferase, mu 1 solute carrier family 39 (metal ion transporter), member 6 GLI pathogenesis-related 1 (glioma) RAS p21 protein activator 2 lymphoid-restricted membrane protein copine III methionine sulfoxide reductase B2 hairy and enhancer of split 6 (Drosophila) -2.04 2.24 2.09 2.43 -4.33 -2.64 -2.28 2.51 -2.27 -3.20 2.12 C920005C14Rik Il2rg Prcp Coro7 Aqp9 Ctse Cdr2 Arl6 Dpy19l1 Wrn Rab27a RIKEN cDNA C920005C14 gene interleukin 2 receptor, gamma chain prolylcarboxypeptidase (angiotensinase C) coronin 7 aquaporin 9 cathepsin E cerebellar degeneration-related 2 ADP-ribosylation factor-like 6 dpy-19-like 1 (C. elegans) Werner syndrome homolog (human) RAB27A, member RAS oncogene family 663 711 729 733 742 755 761 769 51 352 307 170 22 95 60 159 788 92 789 824 859 904 913 236 146 47 425 34 959 964 116 16 1057 1111 403 136 1151 1158 1248 142 351 234 1254 1286 1296 1305 315 46 405 250 1306 1309 1352 1419 1436 1517 1518 145 107 144 225 73 126 271 1553 1574 1594 1624 1632 1693 1705 1745 1767 1790 1791 321 244 304 190 422 376 346 165 345 402 290 2.11 Rhob 3.73 2.75 7.58 P2ry10 Nrgn Cldn12 2.76 B3gnt5 2.61 Pik3cg ras homolog gene family, member B purinergic receptor P2Y, G-protein coupled 10 neurogranin claudin 12 UDP-GlcNAc:betaGal beta-1,3-Nacetylglucosaminyltransferase 5 phosphoinositide-3-kinase, catalytic, gamma polypeptide 1794 293 1802 1835 1843 37 113 7 1848 110 1908 135 Supplementary Table 4. Only 16 out of the 156 downstream targets of HOXB4 identified by Schiedlmeier et al.17 were also observed to be downstream targets of HOXB4 in this report. Furthermore, only one direct downstream target, Rasa2, overlapped in both studies (shown in bold). Gene Symbol Ccr2 Etv5 Gadd45a IL15 Klf3 Narf Nfkbie Osbpl5 Ptgs2 Rasa2 Rassf4 Serpina3g Socs5 Tex9 Tnfaip2 Zfp260 Gene Title chemokine (C-C motif) receptor 2 ets variant gene 5 growth arrest and DNA-damage-inducible 45 alpha interleukin 15 Kruppel-like factor 3 (basic) nuclear prelamin A recognition factor nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, epsilon oxysterol binding protein-like 5 prostaglandin-endoperoxide synthase 2 RAS p21 protein activator 2 Ras association (RalGDS/AF-6) domain family 4 serine (or cysteine) peptidase inhibitor, clade A, member 3G suppressor of cytokine signaling 5 Testis expressed gene 9 tumor necrosis factor, alpha-induced protein 2 zinc finger protein 260 /// similar to Zinc finger protein OZF (Zinc finger protein 260) (Zfp-260) Supplementary Material and Methods ChIP-chip Formaldehyde cross-linked chromatin was prepared from the three KLS-EML-HOXB4 clones and incubated with the I12 anti-human HOXB4 rat monoclonal antibody (Developmental Studies Hybridoma Bank, University of Iowa) that had been previously bound to Dynabeads magnetic beads (Invitrogen) conjugated to sheep-anti-rat IgG2a antibody. As a control, formaldehyde cross-linked chromatin was prepared from KLSEML-HOX4 clone #4 cells and incubated with the isotype control anti-rat IgG2a antibody (sc-53740) (Santa Cruz Biotechnology) that had been previously bound to Dynabeads magnetic beads (Invitrogen) conjugated to sheep-anti-rat IgG2a antibody. ChIP was performed using the Dynabeads magnet system (Invitrogen). The immunoprecipitate (IP) was serially washed eight times using the Dynabeads magnet system (Invitrogen) and supernatants from the serial washings were collected and represent eluates 1-8 in Figure 5. Western blot analysis of ChIP eluates was performed using the anti-HOXB4 (N-18) antibody (sc-15001) (Santa Cruz Biotechnology) and antiActin antibody (sc-1616) (Santa Cruz Biotechnology). Blots were visualized with the HRP-conjugated donkey-anti-goat secondary antibody (sc-2020) (Santa Cruz Biotechnology). Processing and microarray hybridization of the IP samples isolated utilizing the I12 anti-rat human HOXB4 antibody or the isotype control anti-rat IgG2a antibody and control total input DNA samples were carried out by Roche NimbleGen. NimbleGen microarray surface artifacts were removed using the SmudgeKit package from J. Emerson et al. (manuscript in preparation). The 2000 highest scoring HOXB4 binding sites were identified using the Tilescope software,1 using quantile normalization, a window size of 1000, p-value threshold of 2.0, peak interval size of 1000, a feature length of 500 and the iterative peak identification method. These sites showed an enrichment over control of 1.77 fold or greater. 1910 of the 2000 sites were close to RefSeq gene transcription start sites and were used for analysis. The 1000 regions with the strongest binding were used to perform de novo motif discovery of overrepresented 6, 8 and 10mers on both DNA strands using the Weeder software.2 Tilescope software and Weeder software analyses were provided by the Yale Center of Excellence in Molecular Hematology (YCEMH). Quantitative PCR analysis was performed using primers designed to the corresponding gene promoter regions and are available upon request. References 1. Zhang ZD, Rozowsky J, Lam HY, Du J, Snyder M, Gerstein M. Tilescope: online analysis pipeline for high-density tiling microarray data. Genome Biol. 2007;8:R81. 2. Pavesi G, Pesole G. Using Weeder for the discovery of conserved transcription factor binding sites. Curr Protoc Bioinformatics. 2006;Chapter 2:Unit 2 11.