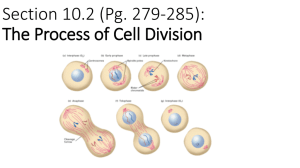
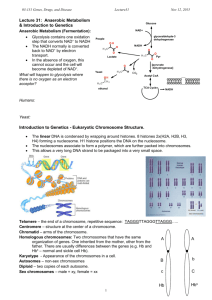
lec03-1
advertisement

Advance Molecular Biology (LS642100, 2003) Part I-2 T. Y. Lin http://www.oup.co.uk/best.textbooks/biochemistry/genesvii/ I. Chromosomes (Chapter 18) 1. DNA is contained in a compact compartment. (1). The density of DNA in the compartment is high. (2). The packaging of chromatin is flexible. (3). The packing ratio: the length of the DNA divided by the length of the unit that contains it. 2. Condensing viral genomes into their coats. (1). TMV RNA becomes coiled in a helical array on the inside of protein shell. (2). Lambda phage DNA packed into a head shell (involving translocation and condensation) (3). Some plant viruses are multipartite. 3. The bacterial genome (1). The lysed E. coli nucleoid is released in the form of loops of a fiber. (2). Whether a DNA-binding protein plays a structural role in the nucleoid? a. Protein HU (hupA,B) (a). HU forms a dimmer that condenses DNA. (b). HU stimulates DNA replication. (c). HU is related to IHF. b. Protein H1 (H-NS) (a). H1 binds DNA, interacting preferentially with sequences that are bent. (b). H1 affects on the local topology of DNA, with effects on gene expression that depends on the particular promoter. (3). Bacterial genome consists of loops (~100) of DNA (~40 kb, 13 m). 4. Loops, domains and scaffolds in eukaryotic DNA. (1). Histone-depleted chromosomes consist of a protein scaffold to which loops of DNA are anchored. 1 (2). Interphase cells possess a nuclear matrix (MAR and SAR). (3). The lack of conservation of sequence in MAR fragments (~70% A·T). (4). Commonly present: cis-acting sites; a recognition site for topoisomerase II (5). DNA fragments that contain MAR can bind to a metaphase scaffold. 5. The contrast between interphase chromatin and mitotic chromosomes. (1). At metaphase, the sister chromatids of a mitotic pair each consists of a fiber (~30 nm d) compactly folded into the chromosome. (2). Euchromatin and heterochromatin. 6. The extended state of lampbrush chromosomes. (1). Lampbrush chromosomes are formed during an unusually extended meiosis (up to several months, in certain amphibians). (2). The lateral loops extrude from the chromomeres at certain positions. (3). The loops are surrounded by a matrix of ribonucleoproteins. (4). The loop is an extruded segment of DNA that is actively transcribed. 7. Transcription disrupts the structure of polytene chromosomes. (1). The polytene chromosomes of D. melanogaster form an alternating series bands (0.05-0.5 m) and interbands. (2). The giant chromosomes are produced by the successive replications of a synapsed diploid pair (up to 1024 C). (3). In situ or cytological hybridization. (4). Some of the bands pass transiently through a puffed state. The puffs are sites where RNA is being synthesized. 8. The eukaryotic chromosome as a segregation device (1). Microtubule organizing centers (MTOCs) (2). Centromere: the flanking regions are rich in satellite DNA sequences. (3). Kinetochore: a darkly staining fibrous object of d. or l. ~400 nm. (4). CEN fragments are used simply to attach the chromosomes to the spindle. (5). The yeast CEN elements 2 a. CDE-I: 9 bp, left boundary. b. CDE-II: a >90% A·T-rich sequence of 80-90 bp. c. CDE-III: an 11 bp sequence, right boundary. (6). Proteins that are necessary for the function of CEN sequences. a. CDE-I is bound by the homodimer CBF1 (for fidelity of segregation). b. CBF3 (240 kD) binds to CDE-III (essential for centromeric function). 9. Telomeres seal the ends of chromosomes. (1). Each telomere consists of a long series of Cn (A/T) m, n>1, m=1-4. (2). Telomerase uses an RNA template to synthesize the DNA repeats and add them to the chromosome ends. (3). The telomere-binding protein TRF2. II. Nucleosomes (Chapter 19) 1. Nucleosomes can inhibit multiple steps in gene transcription (1). Transcription activator binding (upstream factors) (2). Preinitiation complex formation (general factors (TATA-box) and polII) (3). Transcription elongation 2. Nucleosome is the subunit of chromatin. 3. DNA is coiled in arrays of nucleosomes. (1). The monomeric nucleosome contains DNA of the unit length. (2). A core particle (146 bp) and the linker DNA (8-114 bp). 4. DNA structure varies on the nucleosomal surface. (1). Sites for nicking lie at regular intervals along core DNA (DNase I digest). (2). The cutting periodicity coincides with the structural periodicity. 5. The path of nucleosomes in the chromatin fiber .TIF .TIF .TIF . 6. Organization of the histone octamer. (1). All four core histones show a similar type of structure in which three 3 -helices are connected by two loops (histone fold). (2). Each heterodimer binds 2.5 turns of the DNA double helix. (3). Each histone has a globular body that contributes to the central protein mass of the nucleosome. (4). Each histone also has a flexible N-terminal tail, which has sites for modification. (5). Acetylation (or methylation) of lysine or phosphorylation of serine reduces the overall positive charge of a protein. (6). Ser10 of H3 is phosphorylated when chromosomes condense at mitosis. (7). Core histones appear to be acetylated and methylated during S phase. (8). All the phosphate groups are removed at the end of the process of division. (9). The phosphorylation of H1 is catalyzed by the M-phase kinase. (10). The effect of phosphorylating H1 may be to eliminate its effects on local chromatin structure. 7. Reproduction of chromatin requires assembly of nucleosomes. (1). The octamers band diffusely between heavy and light densities, suggesting disassembly and reassembly. (2). Histone acetylation a. Acetylated mononucleosomes enhanced DNase cleavage (60 bp from one end) and transcription factor access. b. The tails might have transient interactions with DNA that make them available for interactions with nuclear proteins. c. Amino acids 16-24 of H4 are bound to a negatively charged face of H2A and H2B on an adjacent nucleosome. (3). Histone acetyltransferase (HAT) a. Cytoplasmic, type A HAT (a). Newly synthesized and acetylated H3 and H4 assembled into a chromatin assembly complex (CAC) with chromatin assembly factor CAF-1 (>5 subunits, 238 kD) (b). H4 Lys5, Lys8, Lys12 4 b. Nuclear, type B HAT (a). Gcn5 acetylates H3 Lys14 and H4 Lys8 and Lys16 (b). Human TAFII250 (part of TFIID) contains HAT activity (4). Histone deacetylases (HD) a. Sequence-specific DNA-binding repressor proteins recruit HD complexes to the promoter region of a gene to make the nucleosomal histones in the deacetylated state. b. Activator binding recruits HAT, histone acetylated. 8. Do nucleosomes lie at specific positions? (1). Nucleosome positioning places restriction sites at unique positions relative to linker sites cleaved by micrococcal nuclease-indirect end labeling. (2). The deposition of histone octamers on DNA is not random with regard to sequence. (3). A·T-rich stretches locate so that the minor groove faces in towards the octamer; G·C-rich stretches place so that the minor groove points out. (4). Translational positioning determines which regions are more accessible. (5). Rotational positioning describes a change in the exposure of DNA on the surface of the nucleosome. 9. Are transcribed genes organized in nucleosomes? (1). The rDNA transcription unit and the SV40 minichromosomes. (2). RNA polymerase is comparable in size to the nucleosome. (3). Genes that are being transcribed contain nucleosomes at the same frequency as nontranscribed sequences. (4). The nucleosomes are temporarily displaced as RNA polymerase passes through, but reform immediately afterward. (5). Transcription destroys the nucleosomal positioning. 10. DNAase hypersensitive sites change chromatin structure. (1). Hypersensitive sites are created by the (tissue-specific) structure of chromatin. 5 (2). Most hypersensitive sites are found only in chromatin of cells in which the associated gene is being expressed. (3). Hypersensitive sites associated with transcription. (4). Hypersensitive sites are found to be associated with promoters, other elements that regulate transcription, origins of replication, centromeres, and sites with other structural significance. 11. Domains define regions that contain active genes. (1). DNase I sensitivity defines a chromosomal domain. (2). A gene becomes relatively susceptible to the enzyme specifically in the tissue(s) in which it is expressed. 12. Heterochromatin depends on interactions with histones. (1). Proteins of the structural maintenance of chromosome (SMC, ATPases) a. Condensins (the control of overall structure): Condensins form complexes that have a core of the heterodimer SMC2·SMC4 associated with other. b. Cohesins (connections between sister chromatids that must be released at mitosis): Cohesins have a similar organization based on the heterodimeric core of SMC1·SMC3. (2). Position effect variegation in Drosophila a. Inactivation spreads from heterochromatin into the adjacent region over a variable distance (epigenetic effect). b. Extension of heterochromatin inactivates genes. c. Telomeric silencing in yeast. (a). Genes translocate to a telomeric location. (b). Yeast mating type is determined by the activity of MAT. (c). HML and HMR are maintained in an inactive form. The silent loci HML and HMR share many properties with heterochromatin. (d). RAP1 is a sequence-specific DNA-binding protein which binds to the C1-3A repeats at the telomeres, and also binds to the cis-acting silencer elements for repression of HML and HMR. (e). RAP1 recruits SIR3/SIR4 that interact with the N-term tails of H3 and H4. 6 (f). SIR3/SIR4 bind to histones H3/H4 and polymerize. (g). The C-terminus of SIR3 has a similarity to nuclear lamin proteins. 13. Global changes in X chromosomes. (1). Constitutive heterochromatin and facultative heterochromatin. (2). X-linked variegation is caused by the random inactivation of one chromosome in each precursor cell (n-1 rule). (3). X-inactivation center (Xic) is a cis-acting locus that contains the information necessary to inactivate all copies of X chromosomes but one. (4). Xic has an element(s) for counting and the Xist gene for inactivation. (5). The Xist RNA coats the X chromosome from which it is synthesized. (6). Following inactivation, the Xist RNA is found only on the inactive X chromosome. X-inactivation is mediated by stabilizing the Xist RNA on the inactive X-chromosome. (7). The features of the inactive X chromosome include a lack of acetylation of histone H4 and methylation of CpG sequences. (8). Silencing of Xist expression is necessary for the active X chromosome. 14. Methylation is responsible for imprinting. (1). The promoters are methylated when the gene is inactive. (2). Spermatocytes display the methylation pattern that is characteristics of mature sperm. Further changes are made after fertilization. In females, the maternal pattern is imposed during oogenesis. (3). Methylation pattern of germ cells: (1) the previous pattern is erased by a genome-wide demethylation; (2) pattern specific for each sex is imposed. (4). Imprinting describes a change in a gene that occurs during passage through the sperm or egg with the result that the paternal and maternal alleles have different properties in the very early embryo. 15. Mode of epigenetic inheritance. (1). Epigenetic changes influence the phenotype without altering the genotype. 7 (2). Epigenetic mechanisms can be divided into two classes. a. DNA may be modified by the covalent attachment of a moiety. b. A self-perpetuation protein state may be established. (3). A protein responsible for epigenetic effects must have some sort of self-templating or self-assembling capacity. (4). Activation of transcription is associated with acetylation in the vicinity of the promoter of the histones (H3 and H4). (5). Inactivity of constitutive heterochromatin may require that the histones be not acetylated. (6). The effects of the Sup35 protein in yeast. a. Wild-type is [psi-], gene sup35 codes for a translation termination factor. b. When a [psi-] strain is crossed with a [PSI+] strain, all of the progeny are [PSI+]: newly synthesized Sup35 protein is converted into [PSI+] state. c. Inheritance of the [psi-] and [PSI+] conditions is epigenetic. d. The C-terminal domain of [PSI+] is sufficient for the activity of the protein. The N-terminal domain is sufficient for formation of the structures that confer the inactive condition. e. Loss of function in the [PSI+] state is due to the sequestration of the protein in an oligomeric complex. Hsp104 is required fro some change in the structure of Sup35 for acquisition of the [PSI+] state. (7). The occurrence of prion diseases corresponds to an epigenetic inheritance. a. A protease-resistant protein (PrPSc) was purified from the active component of the scrapie. The form of the protein in normal brain, PrPc, is sensitive to proteases. b. Both proteins are glycosylated and linked to the membrane. c. The endogenous protein is necessary for an infection. d. Hamster-PrPSc cannot convert mouse-PrPc to PrPSc. e. The conversion of cellular protein into the Sc state requires that the Sc and C proteins have matched sequences. f. The infectious agent is a protein that can adopt multiple conformations, each of which has a self-templating property. 8