Intro to Inorganic Chemistry - Walla Walla Community College
advertisement

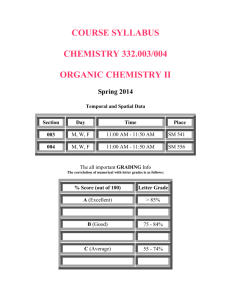
Intro to Inorganic Chemistry Chemistry 121 Instructor: Lecture: Lab: Office: Office Hours: E-mail: Phone: Spring 2009 Pierce College Anna Gorman MWThF 11-11:50 am Cascade 221 T 11-12:50 pm or 1-2:50 pm C204 C204 MWThF 12-1 pm or by appointment annawelk@hotmail.com 253-964-6731 Text and Materials: General, Organic, and Biochemistry by Blei and Odian ISBN: 0-7167-4375-2 Chem 101 Lab Manual Laboratory Goggles Scientific Calculator **A scientific calculator should be brought to each class.** Course Description: Chem 121 is a five-credit course for students how have previously studied chemistry on a little or not at all. This course, together with Chem 131, is designed to meet the needs of students planning to enter careers in the allied health sciences (such as nursing or physical therapy), but it is also appropriate for students who desire a basic one-quarter introduction to chemistry. Instructional Methods: This course will include lectures, demonstrations, along with readings form the text, optional homework problems, laboratory experiments, quizzes, exams, and a comprehensive final. Course Outcomes: Upon successful completion of this course, the student should be able to: 1. list the steps of scientific method 2. employ the metric system in measurements and dimensional analysis with appropriate use of significant figures 3. distinguish between chemical and physical changes 4. explain the atomic nature of matter 5. explain the structure of the periodic table 6. describe the structure of atoms, molecules, and ions in terms of protons electrons, and neutrons 7. predict the shapes and polarities of molecules 8. describe the nature of solids, liquids, gases, and solutions 9. write chemical formulas and balance chemical equations 10. perform calculations using the concepts of moles, reaction stoichiometry, solution concentrations, and the gas laws 11. explain the concepts of electrolytes, acids, bases, and buffers 12. explain and apply the concept of equilibrium 13. use pH in acid/base calculations 14. describe radioactivity, balance nuclear equations, and perform calculations using half-life 15. work safely in the chemistry laboratory as a team member 16. perform and analyze experiments that require precise measurements Lecture Attendance: Lectures must be attended on a regular basis since all the homework, quizzes, and exams are based primarily on lecture material. If you are absent, it is your responsibility to get notes, assignments, and announcements from classmates. Lab: Lab reports will be due at the beginning of the following lab period and will be worth 20 points. More details about this and lab safety will be given later. No lab make-ups. The lowest lab report grade will be dropped at the end of the quarter. In the lab you will work in pairs. Both partners must actively participate in performing every lab experiment and writing every report. Lab reports must have my initials on the data portion of the lab. There will be a weekly 3-7 point pre-lab quiz over information contained in the lab to be preformed. The quiz will be designed to ensure you have pre-read the lab and understand the basics of each week’s lab. They will be given in the first 5 minutes of lab, before the safety talk. No pre-lab quiz make-ups! The lab schedule will be announced in class. Homework: A lot of your learning in the class will directly result from working homework problems. I model some of the questions on tests and exams directly upon assigned homework problems. Homework will not be graded. Quizzes: There will be quizzes throughout the quarter that will NOT be announced ahead of time. No make-up quizzes will be given. Exams: There will be four exams, three chapter exams and one comprehensive final. Each exam will be worth 100 points. No notes or books are allowed. Exam dates are subject to change. Make up tests or quizzes will be allowed only if arrangements are made prior to the regular test date. In the event of an absence due to an emergency, the instructor must be contacted on the day of the test. (You can leave a voice mail!) A makeup exam must be taken before the graded exams are handed back to the class, no exceptions to this rule!!! An automatic deduction of 5% per day will occur for a late exam. Grading: Your grade is based on the percentage of total points you have earned at the end of the quarter according to the following: Final Percentage 100 99 98 97 96 95 94 93 92 91 90 89 88 87 86 85 84 83 82 81 80 79 78 77 76 75 Decimal Grade 4.0 4.0 4.0 4.0 4.0 3.9 3.8 3.8 3.7 3.6 3.5 3.4 3.3 3.2 3.1 3.0 2.9 2.8 2.7 2.6 2.5 2.4 2.3 2.2 2.1 2.0 Final Percentage 74 73 72 71 70 69 68 67 66 65 64 63 62 61 60 59 58 Decimal Grade 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1 1.0 0.9 0.8 0.8 0.7 0.7 0.0 0.0 Academic Integrity: Cheating in any form will not be tolerated. Cheating includes, but is not limited to: copying work or allowing your work to be copied, plagiarism, or use of unauthorized materials on quizzes and exams. Actively looking at another student’s paper during a quiz or an exam is considered cheating and will result in a score of zero for that quiz or exam. ADA Statement: If you believe you qualify for course adaptations or special accommodations under the Americans with disabilities Act, it is your responsibility to contact the Disability Support Services Coordinator and provide the appropriate documentation. If you have already documented a disability or other condition through the Pierce College Disability Support Services Office, which would qualify you for special accommodations, or if you have emergency medical information or special needs I should know about, please notify me during the first week of class. Please contact me by phone at my extension, or schedule an office appointment to meet me in my office hours or at another mutually agreeable time. If my office is not convenient for you, we will schedule an alternate place for the meeting. If you use an alternate medium for communicating, please let me know at least one week before the meeting so that appropriate accommodations can be arranged. Chemistry 121 Homework Assignments And Lab Schedule Suggested Homework Problems Chapter 1: Problems 1-6,8,9-19 Exercises 1,3,7,11,13,15,21,29,33,37,41,47 Chapter 2: Problems 1,3-14 Exercises 1,3,9,10,12,15,17,19,21,23,26-28,33,35-39,41,49,51,53,55,56,58,65 Chapter 3: Problems 1-15 Exercises 5,7-9,11,13,15,17,19,21,23,25,27,29,31,33-35,37,43,45,49,51-53 Chapter 4: Problems 1,3-17 Exercises 1,3,5,7,9,11,13,15,17,19,21,23,27,29,31,33,35,37,39,41,47,49,55 Chapter 5: Problems 1-5 Exercises 1,3,5,11 Chapter 6: Problems 1-5 Exercises 1-5, 7,8,11,13,15,17,19,21,23,27-32,45,49,56 Chapter 7: Problems 1-9,11,12 Exercises 1,3,5,7,15,17,19,21,23,25,29,31,35,51,55,59,65 Chapter 8: Problems 2,4,5,7,8 Exercises 1,5,15,17,21,26,27,39 Chapter 9: Problems 1-10,15 Exercises 5-7,9,11,13,15,17,19,21,23,27,35,51,61,77 Lab Schedule Week 1 Lab Check In and Safety Lecture Week 2 Lab #1 Introduction to Chemistry Laboratory Techniques Week 3 Lab #3 Separation of a Mixture Week 4 Lab #4 Physical Properties of Substances Week 5 Internet Lab Week 6 Lab #6 Identification of Ionic Compounds Week 7 Lab #8 Stiochiometery Week 8 Lab #10 Unlabeled Bottles Week 9 Lab #11 Solutions and Concentrations Week 10 Lab #12 Acid and Base Neutralization Spring 2008 Monday Mar. 30 Class Intro Chem 121 Tuesday Mar. 31 Lab Check In Wednesday Apr. 1 Apr. 6 Apr. 7 Exp #1 Apr. 13 Tentative Schedule Thursday Apr. 2 Friday Apr. 3 Apr. 8 Apr. 9 Apr. 10 Apr. 14 Exp #3 Apr. 15 Apr. 16 Apr. 17 Apr. 20 Exam #1 Ch. 1,2,3 Apr. 21 Exp#4 Apr. 22 Apr. 23 Apr. 24 Apr. 27 Apr. 28 Internet Lab Apr. 29 Apr. 30 May 1 May 4 May 5 Exp. #6 May 6 May 7 May 8 No Classes May 11 May 12 Exp. #8 May 13 Exam #2 Ch. 4,5,6 May 14 May 15 May 18 May 19 Exp. #10 May 20 May 21 May 22 May 25 No Classes May 26 Exp. #11 May 27 May 28 May 29 June 1 June 2 Exp #12 June 3 June 4 June 5 Exam #3 Ch. 7,8,9 June 8 June 9 June 10 Final Exam 12-2 pm June 11 June 12 All assignments, quizzes, and exams are subject to change! Announcements will be made in lecture.