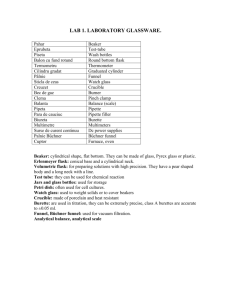
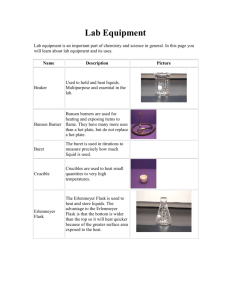
SYNTHESIS NO
advertisement

SYNTHESIS NO.23 Hexaamminenickel (II) Fluoroborate Part I. Synthesis of Ammonium Fluoroborate (NH4BF4). 1. With great care add 20 ml concentrated (~18 M) H2SO4 to 35 ml water in a 250 ml beaker. 2. Add approximately 8 g H3BO3, weighed to the nearest 0.1 g, to the sulfuric acid solution. Heat, with stirring, on a magnetic stirplate under a hood until all the H3BO3 dissolves. With continued stiring, lower the temperature to the point at which the H3BO3 just stays dissolved. 3. With constant stirring slowly add to the H3BO3 solution approximately 20 g NH4F, weighed to the nearest 0.1 g. This reaction is fairly vigorous and should be handled with care. 4. Heat the solution from step 3 on a steam bath under a hood for 30 min, then cool in an ice bath to precipitate ammonium fluoroborate. 5. Suction-filter off the product with a pre-cooled Büchner funnel and wash it with 25 ml of acetone to remove acid. Also pre-cool all tools (stir rods, spatulas, etc.) coming in contact with the solution being filtered. See special sheets on recrystallization for proper washing techniques. Remove the product as soon as possible from the filter paper; keep everything cool. Part II. Synthesis of Hexaamminenickel (II) Chloride 1. Dissolve approximately 12 g of well pulverized NiCl2 • 6H2O, weighed to the nearest 0.01 g, in 40 ml warm water in a 400 ml beaker. 2. Add 50 ml concentrated (~15 M) NH3 to obtain a deep violet solution. 3. Add 60 ml water to redissolve any precipitated product. Stir well. Part III. Synthesis of Hexaamminenickel (II) Fluoroborate. (Part III requires 2 filter flasks. In steps 1 and 2, the first flask is used. The “filtrate” prepared in this first filter flask is then suction-filtered in step 3 into a Büchner funnel attached to the second filter flask.) 1. Weigh 12 g of product from Part I to the nearest 0.1 g in a pre-cooled beaker, then transfer this amount to a clean 500 ml filter flask and dissolve it with 60 ml of water. 2. With a Büchner funnel, suction-filter the solution prepared in step 3, Part II, directly into the solution prepared in step 1. Mix well. 3. Suction-filter off the almost invisible light blue precipitate with a Büchner funnel. Use a small portion of ice-cold 6 M NH3 to rinse the first filter flask thoroughly in order to transfer all of its contents to the Büchner funnel collecting the final product. Using small portions, wash the precipitate with 60 ml ice-cold 6 M NH3, and finally wash it well with acetone to remove the water. The same small portions of 6 M NH3 could be usefully applied to rinsing the walls of the filer flask and transferring the precipitate formed in step 2. Use proper washing techniques. Draw air through the crystal bed to air dry the product, but avoid unnecessary aeration since this will tend to remove NH3 from the product. For the same reason, the product should be transferred to a weighed sample vial and kept stoppered for storage.