De Novo Donor-Specific Human Leukocyte Antigen Antibodies in

H- 01: Antibodies and B-cell biology
H- 14: Acute rejection
De Novo Donor-Specific Human Leukocyte Antigen Antibodies in
Nonsensitized Kidney Transplant Recipients After T Cell-Mediated
Rejection
Chemouny, Jonathan-Maurice 1,2 ; Suberbielle, Caroline 3 ; Rabant, Marion 4 ; Zuber, Julien 5,6,7 ; Alyanakian,
Marie-Alexandra 8 ; Lebreton, Xavier 5,7 ; Carmagnat, Maryvonnick 3 ; Pinheiro, Nathan 4 ; Loupy,
Alexandre 5,6,7 ; Van Huyen, Jean-Paul 4 ; Timsit, Marc-Olivier 6,9 ; Charron, Dominique 3 ; Legendre,
Christophe 5,6,7 ; Anglicheau, Dany 5,6,7,10
Author Information
1 INSERM U1149, Paris, France.
2 Université Paris 7-Denis Diderot, Paris, France.
3 Laboratoire Régional d’Histocompatibilité, Hôpital Saint-Louis, Assistance Publique-Hôpitaux de
Paris, Paris, France.
4 Laboratoire D’Anatomie Pathologique, Hôpital Necker, Assistance Publique-Hôpitaux de Paris, Paris,
France.
5 Service de Néphrologie et Transplantation Adulte, Hôpital Necker, Assistance Publique-Hôpitaux de
Paris, Paris, France.
6 Université Paris Descartes, Sorbonne Paris Cité, Paris, France.
7 RTRS Centaure, Labex Transplantex, Paris, France.
8 Laboratoire D’Immunologie, Hôpital Necker, Assistance Publique-Hôpitaux de Paris, Paris, France.
9 Service D’Urologie, HEGP-Necker, Assistance Publique-Hôpitaux de Paris, Paris, France.
10 INSERM U1151, Paris, France.
Received 19 March 2014. Revision requested 19 April 2014.
Accepted 28 July 2014.
The authors declare no conflicts of interest.
This work was published under the framework of the LABEX TRANSPLANEX [ANR-11-LABX-
0070_TRANSPLANTEX] and benefits from funding from the French government, managed by the
French National Research Agency (ANR), as part of the “Investments for the Future” program.
Correspondence: Dany Anglicheau, M.D., Ph.D., Service de Néphrologie et Transplantation Adulte,
Hôpital Necker-Enfants Malades, 149, rue de Sèvres, 75015 Paris, France.
( dany.anglicheau@nck.aphp.fr
)
Journal : Transplantation
Year : 2015 / Month : May
Volume : 99
Pages : 965
–972 doi: 10.1097/TP.0000000000000448
ABSTRACT
Background
Local inflammation is a potential cause of humoral alloimmune responses in renal transplantation, and de novo donor-specific anti –human leucocyte antigen antibodies (dnDSAs) have been associated with a history of acute rejection.
Methods
We investigated the frequencies and consequences of dnDSAs after a first episode of acute T-cell – mediated rejection (index TCMR) in previously unsensitized kidney transplant recipients.
Results
Of the 1,054 patients who underwent kidney transplantation between September 2004 and December
2010 at our center, we identified 75 unsensitized patients with at least one TCMR. Index TCMRs were diagnosed 4.4 ± 6.8 months after transplantation. The dnDSAs were assessed using the highly sensitive singleantigen human leukocyte antigen bead assay 5.1 ± 3.9 months after the index TCMR and were detected in 16 patients (21%). Patients who developed dnDSAs were more likely to have experienced pre-transplant sensitizing events and were indistinguishable in their clinical, biologic, and histologic variables at the time of index TCMR, although the tubulitis scores tended to be higher ( P =
0.079). These patients experienced a significantly higher incidence of subsequent antibody-mediated rejection episodes ( P < 0.001), but reduced death-censored graft survival was not observed after a median follow-up of 5.5 years post-transplantation. Follow-up biopsies revealed increased antibodymediated changes with significantly higher glomerulitis scores and numerically higher C4d staining scores.
Conclusion
Monitoring anti –human leukocyte antigen antibodies after cellular rejection may be useful, especially among patients with a history of pretransplant exposure to alloantigens, to predict subsequent humoral events and their consequences.
COMMENTS
Sensitive assays for the identification of anti –human leukocyte antigen (HLA) antibodies has improved the identification of donor-specific anti-HLA antibodies (DSAs) for use as key risk factors for poor graft outcomes. Anti-HLA antibodies may arise from blood transfusion, pregnancies, or previous transplantation (preformed DSAs) or from the transplantation itself (de novo DSAs, [dnDSAs]).
Understanding the circumstances surrounding the presence of dnDSAs may help us to avoid their later development in the absence of well-defined strategies.
Most studies focusing on dnDSAs have highlighted an association between early acute T-cell
– mediated rejection (TCMR) and dnDSAs, although the timing of this association remained variable. It has been hypothesized that local mononuclear cell infiltration may cause a breach in humoral tolerance, leading to the appearance of dnDSAs and finally to chronic humoral rejection and graft loss.
In the present study, the authors aimed to investigate dnDSAs using the highly sensitive single antigen
HLA bead (SAB) technique after a first episode of TCMR in a cohort of previously unsensitized, DSAnegative kidney transplant recipients.
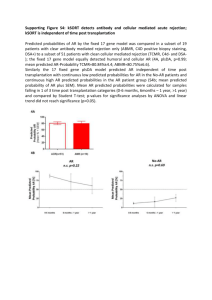
The dnDSAs were detected in 16 of the 75 (21%) patients, nine of which were detected at the time of the index TCMR. The mean time from index TCMR to DSA assay spanned 5.1 ± 3.9 months, The time from renal transplantation to the index TCMR was similar in patients who subsequently developed dnDSAs compared with those who did not ( P = 0.327).
From transplant biopsies the rejection types were similarly distributed between the dnDSA-positive and dnDSA-negative patients ( P = 0.216). However, the mononuclear cell interstitial inflammation (‘i’) score
(2.4 ± 0.5 vs. 2.1 ± 0.7, P = 0.11) and tubulitis (‘t’) scores (2.8 ± 0.4 vs. 2.4 ± 0.8, P = 0.079) tended to be higher in dnDSA-positive patients. Intimal arteritis was similar in the two groups.
The serum creatinine levels 1 year after the index TCMR w ere similar in the two groups (162.1 ± 55.9
μmol/L and 150.5 ± 50.0 μmol/L in the dnDSA-positive and dnDSA-negative patients, respectively, P =
0.400). Because of the increased mortality among patients who developed dnDSAs after the index
TCMR, the mean follow-up after the index TCMR was significantly lower in those patients than in the dnDSAnegative patients (49.9 ± 21.3 months and 65.6 ± 22.7 months, respectively, P = 0.016) This resulted in an increased graft failure, defined as dialysis, a 50% increase from baseline creatinine or death with functioning graft in dnDSA-positive patients ( P = 0.028), whereas death-censored graft failure was similar in both groups ( P = 0.916).
In conclusion, the occurrence of TCMR in previously unsensitized patients triggers the emergence or reactivation of alloimmune donor specific B-cells, DSAs appearance and antibody- mediated rejections without affecting the short-term graft survival unless a repeat rejection occurs. Long-term follow-up studies are required to assess the impact of post-TCMR dnDSAs on long-term allograft survival. These results raise the possibility that monitoring anti-HLA Abs after a first cellular rejection, especially in patients who experienced pretransplant sensitization events, may be useful in predicting subsequent humoral events.
Pr. Jacques CHANARD
Professor of Nephrology