PRAC rapporteur assessment report on the signal
advertisement

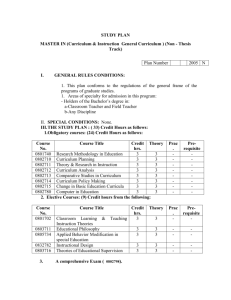
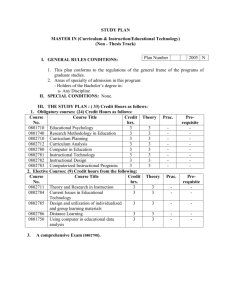
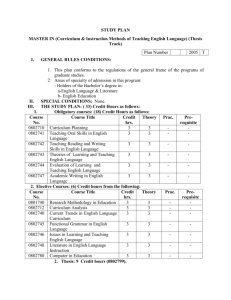
25 June 2013 Pharmacovigilance Risk Assessment Committee (PRAC) <Preliminary/Final> PRAC rapporteur assessment report on the signal of <issue> with <INN and/or product name(s) or product class>1 LEG. no/<xxx> This template should be used by the PRAC rapporteur for signal followup assessments of additional data provided (e.g. MAH submission of cumulative review) based on the PRAC's initial analysis and prioritisation2. NOTE: if the signal follow-up is to be assessed within a regulatory procedure such as PSUR/type-II variation/referral/PASS, etc., the templates for these specific procedures should be used instead. The Annex 'Summary PRAC assessment report' is intended for publication. Delete all drafting notes (like these ones) before finalising this document. For product classes, a footnote should list the INN of the relevant active substances. For some signals, an additional data step may not be required, as available data/evidence may be sufficient to assess the signal following Initial Analysis and Prioritisation step. For those, assessment of the evidence in this template further to EPITT confirmation is recommended. 1 2 7 Westferry Circus ● Canary Wharf ● London E14 4HB ● United Kingdom Telephone +44 (0)20 7418 8400 Facsimile +44 (0)20 7418 8416 E-mail info@ema.europa.eu Website www.ema.europa.eu An agency of the European Union Table of contents 1. Administrative information ...................................................................... 3 1.1. Timetable ............................................................................................................ 3 1.2. Product/class details ............................................................................................. 3 2. Background ............................................................................................. 3 3. Scientific discussion ................................................................................ 4 3.1. PRAC initial analysis and prioritisation ..................................................................... 4 3.2. <NUI results> ..................................................................................................... 5 3.3. Marketing-authorisation holder's responses ............................................................. 5 3.4. Supporting analysis by the EMA or national competent authority ................................ 5 3.5. Rapporteur's position ............................................................................................ 5 4. Conclusion and recommendations ........................................................... 6 5. Points for discussion/agreement............................................................. 6 6. References .............................................................................................. 7 Annex 1: <Title> ......................................................................................... 8 Summary PRAC assessment report on the signal of <issue> with <INN and/or product name(s) or product class> ................................................. 9 [Depending on their length, assessment reports might benefit from a contents page like this, to help the reader easily locate any auxiliary information. Delete if not needed.] <Preliminary/Final> PRAC rapporteur assessment report on the signal of <issue> with <INN and/or product name(s) or product class> Page 2/10 1. Administrative information Active substance (INN) or class name(s) <Text> [Plus invented name, where applicable.] Signal and signal status <Text> <Enter one of these options: Initial analysis and prioritisation2; Follow-up discussion> From <Member State> [Please indicate if Rapp./lead for SM.] PRAC member <Name> <E-mail address> Assessor(s) <Name> <E-mail address> Scientific administrator <Name> <E-mail address> 1.1. Timetable3 Date of report (preliminary/final PRAC <Date> Rapp. AR) PRAC comments on AR <Date> PRAC discussion/adoption <Date> 1.2. Product/class details Strength(s) <Text> Pharmaceutical form(s) <Text> Route(s) of administration <Text> Indication <Text> ATC code <Text> Marketing-authorisation holder(s) <Name(s)> Route of marketing authorisation(s) [Type an 'X' in the relevant box(es) below.] Centrally authorised (or applied for) product(s) (CAPs) Mutual-recognition or decentralised procedure (MRP/DCP) Purely nationally authorised product(s) (NAPs) Other (compassionate use, traditional herbal use, etc.): <Please specify> 2. Background <Text here.> [The section on background provides the context for the assessment, including the therapeutic setting, current regulatory position and relevant regulatory history, and previous submissions and decisions 3 Dates should be entered in the format 'D Month YYYY', e.g. '8 February 2013'. <Preliminary/Final> PRAC rapporteur assessment report on the signal of <issue> with <INN and/or product name(s) or product class> Page 3/10 relevant to the issue. Reference can be made to annexes, including relevant sections of SmPCs, but only if relevant to the assessment subject; it is not helpful to include long annexes if only very small parts are relevant. The therapeutic setting can include the importance of the medical condition relevant to the issue being assessed, cite relevant national guidelines, and comment on relevant clinical practice.] 3. Scientific discussion <Text here.> [All data that have bearing on the issue should be mentioned in this section. It is helpful to specify the methods used for gathering relevant data. If several data sources are used, then an overview of the reviewed data can be included so that the reader forms a clear picture of the scope of the data collected. Thought should be given to the order of presenting data. Many options are available (e.g. categorisation as efficacy data and safety data, or partitioning according to the source of the data, or arranging in chronological order). Generally, the method for ordering data will depend on the issue being assessed and should take into account specific guidance for the type of assessment being undertaken. Supportive and opposing data should be included under each category. A description of the data should be followed by a short critique discussing their strengths, weaknesses and validity. Data evaluation might also include expert opinion. Assessor's commentary on the data can be enclosed in a commentary box to clearly distinguish it from the data.] 3.1. PRAC initial analysis and prioritisation <Text here.> [Insert PRAC initial analysis and prioritisation for a clear notion of the assessment issue, why it has arisen, the scope of the assessment, and the decisions needed — this can be copied from the PRAC recommendation (if already agreed by the PRAC at a previous meeting).] 3.1.1. Published literature <Text here (if applicable).> 3.1.2. Clinical-trial data <Text here (if applicable).> 3.1.3. Spontaneous reports <Text here (if applicable).> <Preliminary/Final> PRAC rapporteur assessment report on the signal of <issue> with <INN and/or product name(s) or product class> Page 4/10 3.2. <NUI results> <Text here (if applicable).> [Provide summary of NUI responses if applicable and as recommended by the PRAC in the initial analysis and prioritisation.] 3.3. Marketing-authorisation holder's responses <Text here.> [Give a short summary of the nature and number of responses received.] 3.3.1. Published literature <Text here (if applicable).> 3.3.2. Clinical-trial data <Text here (if applicable).> 3.3.3. Spontaneous reports <Text here (if applicable).> 3.3.4. Other data <Text here (if applicable).> 3.4. Supporting analysis by the EMA or national competent authority <Text here (if applicable).> [This paragraph should include other studies performed after the PRAC initial analysis and prioritisation that do not fall under one of the above categories, such as: feasibility studies in THIN, GPRD, EudraVigilance supporting analysis.] 3.5. Rapporteur's position <Text here.> [The section on discussion should bring together different strands of the assessment. Drawing on the commentary in the data section, the discussion should identify the effect of the overall data on the issue being assessed, critically evaluating validity and limitations of all the data considered. Where relevant, the data should be discussed in a wider therapeutic context. For some types of assessment, it is helpful to discuss separately all the risks and all the benefits, and then put them together to form an overall view of the balance between risks and benefits. Discussion should offer reasons for any divergence in the data and should attempt to reconcile differences. Areas of uncertainty <Preliminary/Final> PRAC rapporteur assessment report on the signal of <issue> with <INN and/or product name(s) or product class> Page 5/10 should be highlighted. Discussion of the pros and cons of available regulatory actions should be included if relevant.] 4. Conclusion and recommendations <Text here.> [Conclusions should be drawn on the basis of the previous sections of the report. Ideally, they should be shown as numbered, concise points with brief reference to the supporting data. If a specific point remains unresolved then this should be mentioned. Recommendations should state what specific actions can a result of the assessment. Each recommendation should carefully worded to remove any ambiguity about ensuing Ideally, the recommendations should stand out from the text. be considered as be numbered and actions. body of the Each recommendation should be captured in a concise statement; recommendations should not include background reasoning or discussion. If a range of options is available then it may be necessary to present a list of questions for consideration. If a change is recommended to the advice for health professionals or the public, then consider including draft wording. The wording should clearly convey the 'what?', 'how?', and 'when?' for any action required by the health professional or a member of the public.] 4.1.1. Changes to the product information <Text here (if applicable).> 4.1.1.1. Summary of product characteristics (SmPC) <Text here.> 4.1.1.2. Package leaflet <Text here.> 4.1.2. Request for supplementary information <Text here (if applicable).> 4.1.3. Communication <Text here (if applicable).> 5. Points for discussion/agreement <Text here.> <Preliminary/Final> PRAC rapporteur assessment report on the signal of <issue> with <INN and/or product name(s) or product class> Page 6/10 [This section should highlight the areas that need to be considered by the PRAC.] 6. References <Text here.> [A consistent style for references and citations should be used throughout the report. When referencing electronic publications, the date on which the publication was accessed should be shown after the web address. The Agency's standard style for citing (in the body of the report) and referencing (the full source in a list) is illustrated below: The effect of magnesium in myocardial infarctions has been reviewed (Woods, 1991 [1]). Ten years later, a number of recommendations were made to prevent the injection of vinca alkaloids by the intrathecal route (Woods, 2001 [2]). Woods, K.L., 'Possible pharmacological actions of magnesium in acute myocardial infarction', Br J Clin Pharmac, 32, 1991, pp. 3–10. Woods, K, 'The prevention of intrathecal medication errors: a report to the Chief Medical Officer', London, Department of Health, 2001 [cited 11 May 2009]. Available from: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH _4065044 [Accessed 11 May 2009]. Specific referencing software may be used, if available.] <Preliminary/Final> PRAC rapporteur assessment report on the signal of <issue> with <INN and/or product name(s) or product class> Page 7/10 Annex 1: <Title> <Text here.> [Delete this annex if not applicable. Additional annexes can be added if needed.] <Preliminary/Final> PRAC rapporteur assessment report on the signal of <issue> with <INN and/or product name(s) or product class> Page 8/10 25 June 2013 Pharmacovigilance Risk Assessment Committee (PRAC) Summary PRAC assessment report on the signal of <issue> with <INN and/or product name(s) or product class>4 The summary shall not exceed two A4 pages. The summary PRAC assessment report (SmAR) is intended for publication with recommendations for amendments of the SPC/PL on the EMA website. The SmAR focuses on the outcome of the assessment and regulatory comments, taking into account the regulatory and clinical context. Trigger for current safety review, evidence assessed and public-health impact Please provide the trigger for this signal and the evidence leading to the requested changes to product information and the importance of this signal for public health. The signal was raised following a new publication, in the framework of routine pharmacovigilance activities, or following a signal from other regulatory authorities. The Pharmacovigilance Risk Assessment Committee (PRAC) assessed the following evidence/data (i.e. spontaneous/published cases, meta-analysis, data submitted by the marketing authorisation holders, additional data provided in the context of supporting analysis in THIN, and GPRD data). The assessed evidence provides the scientific motivation to request changes to the product information. Patient population/exposure and reaction severity, among other elements, should be taken into account when discussing public-health impact. 4 For product classes, a footnote should list the INN of the relevant active substances. 7 Westferry Circus ● Canary Wharf ● London E14 4HB ● United Kingdom Telephone +44 (0)20 7418 8400 Facsimile +44 (0)20 7418 8416 E-mail info@ema.europa.eu Website www.ema.europa.eu An agency of the European Union © European Medicines Agency, 2016. Reproduction is authorised provided the source is acknowledged. Changes to the product information Summary of product characteristics (SmPC) <Text here.> Please provide the wording of the relevant sections of the SmPC. Package leaflet <Text here.> Please provide the wording of the relevant sections of the package leaflet. Timelines for implementation <Text here.> Please provide the timelines for implementation of the changes to the product information as agreed by the PRAC. Summary PRAC assessment report on the signal of <issue> with <INN and/or product name(s) or product class> Page 10/10