PS4 - MS-IR-UV-NMR - Chemistry at Winthrop University
advertisement
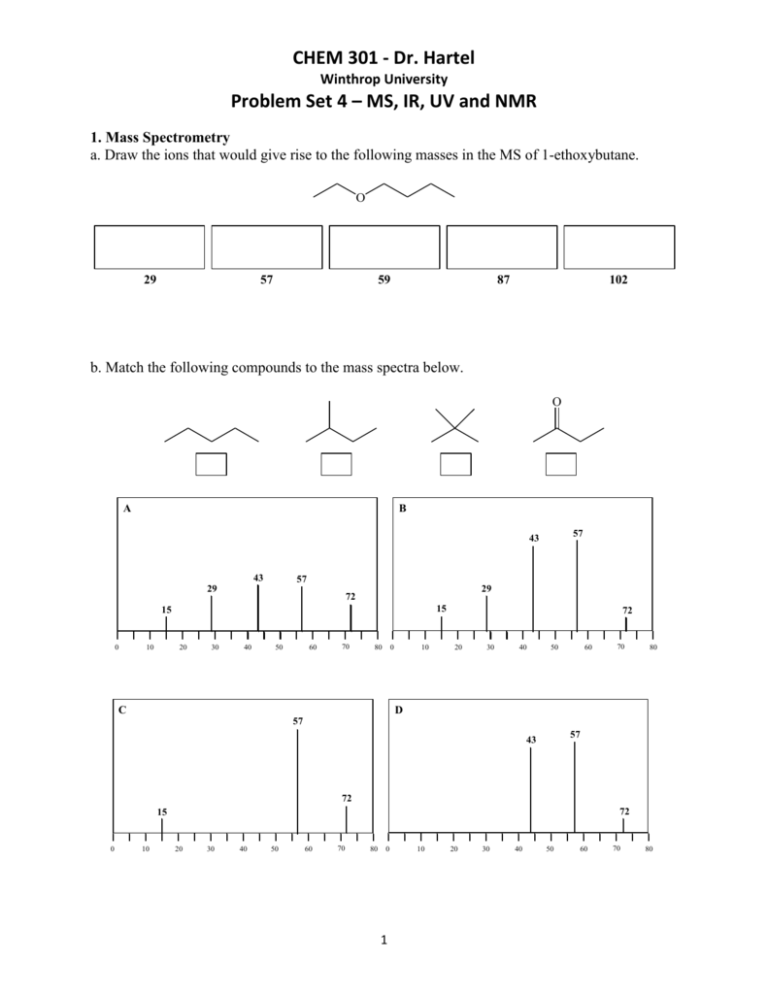
CHEM 301 - Dr. Hartel Winthrop University Problem Set 4 – MS, IR, UV and NMR 1. Mass Spectrometry a. Draw the ions that would give rise to the following masses in the MS of 1-ethoxybutane. O 29 57 59 87 102 b. Match the following compounds to the mass spectra below. O A B 57 43 43 57 29 29 72 15 15 0 10 20 30 40 50 60 70 80 0 C 10 72 20 30 40 50 60 70 80 D 57 57 43 72 72 15 0 10 20 30 40 50 60 70 80 0 1 10 20 30 40 50 60 70 80 CHEM 301 - Dr. Hartel Winthrop University Problem Set 4 – MS, IR, UV and NMR c. The MS of the following molecule gives mass fragments of 139, 125 and 111. Rank the fragments in order of decreasing intensity. d. Which of the following compounds will have an exact mass of 120.0940 amu? F OH O O O O e. Identify the compound with an exact mass of 88.0888 amu and the LRMS below. 70 45 73 88 0 10 20 30 40 50 2 60 70 80 90 100 CHEM 301 - Dr. Hartel Winthrop University Problem Set 4 – MS, IR, UV and NMR 2. Infrared Spectroscopy a. Which signal would be more intense in an IR spectrum, a C-O stretch or a C-N stretch? Why? b. Which would give a stronger C=C stretch in an IR spectrum, 1-butene or 2-butene? Why? c. Which would have a higher frequency absorption, an N-H bond or a P-H bond? Why? 3 CHEM 301 - Dr. Hartel Winthrop University Problem Set 4 – MS, IR, UV and NMR d. Identify the following spectra as cyclohexanone, 2cyclohexen-1-ol or cyclohexene. IR Spectra from SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology, 10/5/09) 4 CHEM 301 - Dr. Hartel Winthrop University Problem Set 4 – MS, IR, UV and NMR 3. Ultraviolet Spectroscopy a. Circle the molecules that would show an absorption in a UV spectrum O O O O OH b. Circle the molecules that would show multiple absorptions in a UV spectrum. O O c. Order the following molecules in decreasing order of λmax (1 highest to 4 lowest). d. Which molecule above would have the most intense absorption in a UV experiment? 5 CHEM 301 - Dr. Hartel Winthrop University Problem Set 4 – MS, IR, UV and NMR 4. NMR Spectroscopy a. If an average carbon requires irradiation at 75 MHz to resonate in a 7.046 T magnetic field, what frequency will be required to resonate an average carbon in a 14.092 T magnetic field? b. Use the following molecule to answer the following. a c b d Cl Which carbon is furthest downfield? Which carbon is furthest upfield? Which carbon resonates at the highest frequency? Which carbon resonates at the highest chemical shift? Which carbon resonates at the lowest chemical shift? Which carbon is the most shielded? Which carbon is the most deshielded? ___________ ___________ ___________ ___________ ___________ ___________ ___________ c. For each of the following molecules, indicate the number of signals (resonances) that would be observed in a 13C-NMR experiment. O Br OCH3 O Cl Cl 6 CH3 CHEM 301 - Dr. Hartel Winthrop University Problem Set 4 – MS, IR, UV and NMR d. Determine the structure of a compound with a formula of C5H10 and the following 13C-NMR. 140 120 100 80 PPM 60 40 20 0 e. For each of the following molecules, indicate the number of signals (resonances) that would be observed in a 1H-NMR experiment. O Br OCH3 O Cl Cl 7 CH3 CHEM 301 - Dr. Hartel Winthrop University Problem Set 4 – MS, IR, UV and NMR f. For each of the following molecules, indicate the multiplicity (splitting) expected for each hydrogen type in a 1H-NMR experiment. O F O O Cl HO CH3 g. In scientific literature, 1H-NMR data is reported in the following manner: δ CS (m, J = X Hz, NH) where “CS” is the chemical shift for a resonance “m” is the peak’s multiplicity “X” is the peak’s coupling constant (if any) “N” is the number of hydrogens represented. Draw the 1H-NMR spectrum using the following data (ignore the J values): δ 3.65 (t, J = 5.0 Hz, 2H), 3.24 (s, 3H), 2.62 (t, J = 5.0 Hz, 2H), 2.09 (s, 3H). Determine the structure of a ketone with the formula C5H10O2 that is consistent with this data. 8