Drugs That Relieve Pain:
advertisement

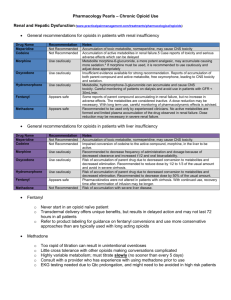
Drugs That Relieve Pain: Opioid and Nonopioid Analgesics There are three types of available analgesics 1. Opioid (narcotic) analgesics 2. Nonopioid (nonnarcotic) analgesics 3. Other drugs which act as adjuvants (Agents that aid or increase the action of the principle drug or that affect the absorption, mechanism of action, metabolism, or excretion of the primary drug in such a way as to enhance its effects.) when taken with other opioid or nonopioid analgesics. Sometimes these drugs may also have analgesic activity of their own. These drugs include certain antidepressants and antimanic drugs. Opioid Analgesics Opiods are said to be irreplaceable in medicine as the most important drugs that relieve severe pain. Pain is usually associated with actual or potential tissue damage. When body tissue is damaged, small-diameter sensory (afferent) fibers of peripheral nerves get activated. They are called nociceptive sensory neurons and are located on our skin, muscles, joints and abdominal viscera. The synaptic terminals (where the action potentials are conducted) are located in the dorsal horn of the spinal cord, which is also where the release of such chemical neurotransmitters as neuropeptide substance P, nerve growth factor, neurotropic factors, and the amino acid neurotransmitter glutamate are induced. The release of pain-signaling neurotransmitters is regulated by endogenous endorphins and/or by any opioid agonist, since it mimics the analgesic action of our endogenous endorphins, acting upon the same set of receptors. So, opioids and endorphins exert presynaptic inhibition on the terminals of afferent sensory neurons to inhibit the release of pain-signaling neurotransmitters. History Opium is extracted from poppy seeds (Paper somniforum). It has been used for thousands of years all around the world to produce euphoria, analgesia, sleep and relief from diarrhea and cough. Recreational abuse and addiction to opium have been recorded as early as pre-Christian centuries (noted by writers such as Homer, Hippocrates, Disorides, Virgil, and Ovid). In the 1800’s, opium addiction and abuse was the reason for China’s defeat in the Anglo-Chinese War, also known as the Opium War. From the early Greek and Roman times up until 16th and 17th centuries, medicinal and recreational uses of opium were well established. People used to combine it with alcohol (the mixture known as laudanum, meaning “something to be praised”), and referred to it as the “stone of immortality”. Opium and Laudanum were used to treat practically every known disease. In the early 1800’s, morphine was isolated from opium as its active ingredient. Since then, morphine has been used around the world as the leading agent for treating severe pain. By 1910, concerns began to mount about the danger of opioids and the dependence that it may induce. In 1914, the Harrison Narcotic Act was passed, regulating and controlling the use of most opioid products. Terminology Before learning more about opioids, it helps to know the definitions of certain terms: Analgesia- a state of insensitivity to pain, even though the subject is fully conscious Opium- A Greek word that means juice, more specifically the juice that is extracted from the poppy Papaver somniferum. Opiate- a drug that is extracted from the poppy, this term is restricted to two drugs that are naturally found in opium, morphine and codeine. Opioid- any exogenous drug (natural or synthetic) that binds to an opiate receptor and produces agonist, or morphine-like effects. Opioid Antagonist- any drug that binds to an opiate receptor and antagonizes the effects of morphine, displacing it from the receptor. Mixed agonist-antagonist opioids- have a weak analgesic action at opiate receptors, but precipitate withdrawal in opioid-dependent persons. Endorphin- generic, all-inclusive term that applies to any endogenous substance (naturally formed in the body) that exhibits pharmacological properties of morphine. There are three families of endogenous opioid peptides: Enkephalins- 2 types that have the same 4 of the five amino acids: Tyrosine-glysineglycine-phenylalanine. Leu-enkephalin terminates in a leucine and Met-enkephalin terminates in methionine. Dynorphins- These are the least understood of the endogenous opioids. They are cleaved from prodynorphin and have a similar distribution to the enkephalins, but are only very weak analgesics. The precise function of dynorphins is uncertain, but they have a high affinity for k receptors, which mediate many of the unpleasant dysphoric side effects of opioids. Beat-endorphins- A peptide consisting of amino acid sequence 61-91 of the endogenous pituitary hormone beta-lipotropin. The first four amino acids show a common tetrapeptide sequence with methionine- and leucine enkephalin. The compound shows opiate-like activity. Injection of beta-endorphin induces a profound analgesia of the whole body for several hours. Narcotic- originally derived from Greek word narke, meaning “numbness”, “sleep”, or “stupor”. Originally referring to any drug that induced sleep, the term later became associated with opioids, such as morphine and heroin. Opioid Receptors There is a general agreement on the existence of at least three types of opioid receptors: mu, kappa, and delta. They are all differentially distributed throughout the CNS; the clinical significance of this distribution is still unclear. All opioid receptors belong to a superfamily of G-protein-coupled receptors, all of which possess seven membrane-spanning regions. Each receptor type arises from its own gene and is expressed through a specific messenger RNA (mRNA). Each receptor is a chain of about 400 amino acids. About 60% of the amino acid sequences are identical in all three receptors and about 40% are different. That diversity is responsible for the specific fit of an endogenous endorphin or exogenous opioid to a specific receptor. Mu Receptors: It seems to display the best and strongest analgesic actions, but also the highest abuse liability. There are two types of Mu Receptors: mu-1 and mu2. Mu-1 receptors are located outside the spinal cord and are responsible for the central interpretation of pain. Mu-2 receptors are located throughout the CNS and are responsible for respiratory depression, spinal analgesia, bradycardia (slowness of the heart beat), physical dependence and euphoria. Mu receptors are present in all structures in the brain and spinal cord involved in morphine-induced analgesia. They are also present in brain-stem nuclei involved in control of respiration and in morphine’s depression of respiration, in brain-stem structures involved in initiation of nausea and vomiting, and in the nucleus accumbens, an area involved in the compulsive abuse of opioids, behavioral stimulants and other drugs subject to abuse. Kappa Receptors: Found in high concentrations in the basal ganglia, nucleus accumbens, ventral tegmentum, deep layers of cerebral cortex, hypothalamus, and dorsal horn of spinal cord. Kappa receptors may antagonize mu-receptor activity. They produce modest amounts of analgesia, dysphoria, disorientation, depersonalization feelings, and mildrespiratory depression. Dynorphin is the endorphin with the greatest affinity for the kappa receptors. Delta Receptors: Thought to modulate the activity of mu-receptors. The enkephalins are the endogenous endorphins for the delta receptors. They are responsible for analgesia at both the spinal and brain levels. Delta receptors are found in nucleus accumbens and limbic system, possibly playing a role in the emotional responses to opioids. Pure Agonists- a drug that binds to a receptor and changes the characteristics of the natural ligand. Opiate examples of this would include: -Morphine- found in the opium poppy, and is the most clinically used for treating pain. -Methadone-usually taken orally, long acting, used to treat opioid addiction -Fentanyl- short acting used clinically in anesthesia. Crosses the Blood Brain Barrier faster than morphine -Codeine- common clinically used to treat moderate pain, and a cough suppressant. Pure Antagonists- a drug that binds to a receptor but no change in the characteristics of the cell occur. They also block the ability of natural ligands and other drugs to bind at these sites. Antagonists may be used to trigger withdrawal symptoms in dependent individuals. -Naltrexone- When administered to opioid addicts, if an opioid is taken, it will block the analgesic and euphoric effects. -Naloxone- displaces morphine from mu receptors and blocks analgesic effects. It also does not trigger feelings of dysphoria, or pain. Mixed Agonist-Antagonists- a drug that produces an agonist effect at one receptor site, and antagonist effects at another site. These drugs are not as efficacious for treating pain as a pure agonist. -PentazocinePartial Agonists- a drug that binds to opioid receptors with a low efficacy, and therefore have a lower maximal effect than a pure agonist. -Buprenorphine- binds to all three types of opioid receptors and can produce analgesic effects when given to an opioid naïve person, but trigger withdrawal symptoms in tolerant persons. Pharmacokinetics of Morphine -Administered orally, rectally, or by injection. It is also injected into the spinal canal during labor to avoid transferring the drug across the placenta, and for long term use to avoid the side effects. -Morphine is more water soluble so it is slower to cross the blood brain barrier. Usually only 20% of the dose will reach the CNS. -It is metabolized by the liver into its metabolite morphine-6-glucuronide, which is 10-20 times more potent than morphine. -The Half-life is about 3-5 hours. Pharmacological Effects of Morphine Analgesia: -produces an indifference to pain by reducing the intensity of the pain and distress without becoming unconscious by changing the central processing of pain. Naloxone can be used to displace morphine and block the analgesic effects. Euphoria: -Morphine produces a reinforcing euphoric, pleasant state of well being and lack of concern. When used repeatedly tolerance develops to these effects. Opioids activate the mesolimbic dopamine reward system which include the Ventral Tagmental Area (VTA) and the Nucleus Accumbens (NA). The activation of these areas are believed to be responsible for the rewarding effects of cocaine, alcohol, and benzodiazepines. Respiratory Depression: -Morphine decreases the respiratory center’s ability to detect higher levels of carbon dioxide in the blood. With higher doses, the respiratory volume is lowered, and breathing becomes irregular or shallow and eventually cease. This is the largest concern with opioid overdose. Cough Suppressant: - Opioids suppress a part of the brain stem involved in the cough reflex. Codeine is the most popular for this effect. Other Pharmacological effects: -Causes papillary constriction (miosis). -Nausea and vomiting -relieve diarrhea -release histamine resulting in localized itching or sever allergic reactions. Tolerance and Dependence -Opioids are limited in their use because of the tolerance that develops and the possible abuse potential. -Opioids produce cross tolerance. -Withdrawal results in reduced dopamine release in the NA, and an increase in the release of norepinephrine. The symptoms of withdrawal are usually the opposite of the effects while taking the drug. The magnitude depends on the frequency, dose, and time the drug was used. -A new technique has been developed to aid in opioid withdrawal, called Rapid Opioid Detoxification where a pure opioid antagonist (Naloxone) and a sympathetic blocker clonidine are administered while under general anesthesia. This allows total detoxification within a few hours instead of a few weeks. Naloxone administration is continued to decrease cravings, and psychotherapy is offered as support to avoid relapse. Treatment is also provided for any other comorbid diagnosis. List of Drugs You should know Other Pure Agonist Opioids 1) Codeine a) Occurs naturally in opium b) One of the most commonly prescribed drugs c) Is combined with Tylenol or Acetaminophen to relieve mild to moderate pain d) Available OTC in Canada i) Must have 2 other active ingredients… (1) Caffeine (2) Acetaminophen e) ~40% of people who use them meet the criteria for codeine dependence i) Use of codeine containing products is strongly correlated with endogenous opioid depression f) Pharmacokinetics of Codeine i) Converted to morphine via CYP2D6 enzyme (hepatic). ii) Thought that the effects of pain relief are induced by actions of morphine, not the codeine itself. (1) Serotonin Specific Antidepressants block the conversion of codeine to morphine (a) In doing so, block the analgesic effects 2) Heroin (Diacetylmorphine) a) A.K.A. Smack, Junk, Black Tar, etc… b) 3x’s more potent than morphine i) made from a slight modification of morphine’s chemical structure (1) Makes it more lipid soluble (a) *Morphine and Heroin are equally effective when the get to the brain, but since latter is more lipid soluble, it crosses the blood-brain barrier quicker, thus causing an intense rush* c) Pharmacokinetics of Heroin i) HeroinMonacetylmorphine Morphine (1) See diagram d) Heroin is legally available in Canada and the United kingdom for clinical use i) Not legal in the U.S. but is widely used illicitly e) Cocaine + Heroin smoked together = i) Intensified euphoria ii) A decrease in paranoia and anxiety that is associated with cocaine (1) Creates a multi-drug addiction that is difficult to treat f) Misc. i) Speedball (1) An intravenous injection of cocaine and heroin together ii) Chui Lung (Chasing the Dragon) (1) Smoking heroin from a piece of aluminum foil (a) Spiral of smoke looks like the tail of a dragon 3) Meperidine (Demerol) a) A synthetic opioid i) Thought to be free of the undesirable properties of opioids due to its structural difference (1) However it is addictive and can be substituted for morphine and heroin in those addicted ii) Side effects differ than that of morphine (1) Exhibits more excitatory effects from its metabolite, normerperidine (a) Tremors (b) Delirium (c) Hyperflexia ( a rxn to the ANS caused by over stimulation) (d) Convulsions 4) Methadone (Dolophine) a) A synthetic mu agonist opioid i) Pharmacological activity is similar to that of morphine b) The principal agent used for prevention of withdrawal symptoms seen in the opioid dependent individual (Methadone Clinics) i) Blocks effects of Heroin withdrawal ii) Introduced as a substitute in 1965 c) Statistics i) Where liberal doses are used, ~1/3 of the clients experience withdrawal (Nonholders) ii) 2/3 (holders) don’t experience withdrawal during a once-daily dosing schedule (1) In order to maintain compliance, prescribers must be free to regulate doses to meet individual requirements 5) Fentynal (Sublimaze) a) Also 3 other related compounds i) Sufenta ii) Alfenta iii) Ultiva (1) All of these are shorts-acting IV agonists b) Fentynal i) Available in (1) Transdermal skin patch (2) Allows prolonged, steady levels of drug concentration in the blood (3) Lollipop (4) Used for short term treatment of surgical pain in children (5) China White (a) When Fentanyl is used illicitly c) All 4 of these drugs are 80-500x’s more potent than morphine as analgesics i) They greatly depress respiration 6) Remifentanyl a) Shortest half life of any known opioid i) 10-20 minutes ii) Rapidly metabolized by enzymes in blood iii) IV administration to control brief, intense pain during surgery Partial Agonist Opioids 1) Burprennorphine (Buprenex) a) Semisynthetic partial agonist opioid b) Axn is characterized by its ceiling effects i) Limited stimulation of mu receptors (1) Responsible for its analgesic effectiveness (2) Inducement of of euphoria and respiratory depression c) High affinity for binding to mu receptors i) Limits its reversibility by drugs like naloxone d) Because of of the ceiling effect, doses 4x’s the daily maintenance dose could be given twice weekly w/o either agonist effects or withdrawal symptoms occurring e) Uses i) Used as an alternative or subsequent step to methadone for opioid detoxification and maintenance programs ii) Used as a “tapering medication for rapid opioid detoxification followed by opioid antagonist therapy 2) Tramadol (Ultram) a) Exhibits a unique dual analgesic action i) Partial agonist at the mu receptors ii) Blocks the presynaptic reuptake of Norepinephrine and 5HT (1) i.e. it produces an antidepressant as well as analgesic effect b) Metabolism i) Tramadol mono-demethyl tramadol (1) The first metabolite is as active or more active than the parent compound c) Partial Agonist effect i) Exhibits a ceiling effect on analgesic (1) Limits respiratory depression (2) Limits abuse potential d) *CONCERN* i) Tramadol + 5HT Type antidepressants (1) Combination of these drugs can lead to Serotonin Syndrome Mixed Agonist-Antagonist Opioids 1) There are four of them a) Pentazoicine b) Butophenol c) Nalbuphine d) Dezocine 2) These drugs are weak mu agonists a) Their limited analgesic effectiveness results from stimulation of the kappa receptors b) Low doses Moderate analgesia c) High Doses little additional analgesia 3) These drugs to precipitate withdrawal in the opioid dependent individual 4) Adverse side effects a) Dysphoria b) Anxiety c) Hallucinations 5) Prototypical Mixed Agonist-Antagonists a) Pentazocine (Talwin) i) T’s and Blues (Talwin + Tripelannamine, an antihistamine) (1) Increase in abuse over past years (2) Causes (a) Seizures (b) Psychotic episodes (c) Skin Ulcerations (d) Abscesses (e) Muscle Wasting b) Butorphenol (Stadol) i) Became available as the first analgesic nasal spray in 1993 (1) Use of nasal Spray Euphoria Abuse 6) Nalbuphine (Nubair) i) Primarily a kappa agonist of limited analgesic effectiveness ii) Also a mu antagonist iii) Not likely to cause respiratory depression or abuse 7) Dezocine (Dalgan) i) The newest of the agonist-antagonist drugs ii) Moderate mu agonist iii) Weak delta and kappa agonist iv) Can substitute for morphine Antagonist Opioids 1) 3 clinically available drugs a) Naloxone b) Naltrexone c) Nalmefene i) All 3 are structural derivatives of Oxymorphone ii) They have affinity but exert no efficacy 2) Naloxone (Narcon) a) Is the prototype antagonist b) Little or no effect when injected into non-opioid dependent individuals c) Rapidly precipitates withdrawal when injected in opioid dependent individuals d) Not absorbed by G.I. tract i) Must be IV administered e) Limitting factor i) Duration of action is short, 15 to 30 minutes (1) Must be reinjected at short intervals to avoid “renarcotization” f) Used to reverse… i) Respiratory depression that follows acute opiod intoxication (overdosing) ii) Respiratory depression in new borns of opioid dependent moms 3) Naltrexone (Trexan, ReVia) -1925 a) The 1st orally absorbed pure opioid antagonist b) Axn’s resemble naloxone but… i) Is well absorbed through oral administration ii) Has a long duration of action (1) Single daily dose of 40-80mg c) Used i) Clinically in treatment programs when it is desirable to maintain a person on chronic therapy w/ an opioid antagonist rather than an opioid agonist such as methadone ii) In treatment of alcoholism to reduce the craving for alcohol during the maintenance period of treatment d) Limitting factors i) Nausea ii) Dose-dependent liver toxicity (1) Can be a problem in patients with preexisting liver disease e) Cognitive-Behavioral Therapy + Naltrexone i) Less drinking ii) Longer time to relapse (1) More time to relapse than did CBT+ Placebo treated patients iii) Effects of this combination seem synergistic f) Naltrexone also has some efficacy in treatment of i) Self-Injurious Behaviors ii) Borderline Personality Disorder iii) Autism (1) Does not treat any core etiology of autism (2) Reduces… (a) Hyperactivity (b) Irritablility (c) Self-injurious behaviors (d) Can be used to maintain a high level of endogenous opioids (i) Prevents a decrease in endorphins (ii) Experience euphoria 4) Nalmefene (Revex) a) Injectable pure opiod antagonist b) Half life of 8 to 10 hrs i) Longer than naloxone ii) Incidence of renarcotization is greatly reduced c) Used to reverse respiratory depression in Overdosing i) If administed to an addict, the precipitation of withdrawal can be prolonged and require additional medical treatment Pharmacotherapy of Opioid Dependence 1) In any treatment of opioid dependence, pharmacotherapy is the foundation, involving more than just methadone a) LAAM and/or Buprenorphine for treatment resistant individuals or Nonholders b) Opioid antagonist therapy for individuals for whom it is more appropriate than agonist therapy c) Treatment of commorbid disorders, especially affective disorders i) e.g. Depression 2) Nonpharmacological services are also needed for success, but how much? a) Large amounts of support to methadone-maintained patients were not costeffective b) Moderate amounts of support are better than minimal amounts i) Should look like a bell curve 3) Duration of methadone maintenance a) Felt that total abstinence from all opioids need not be an objective for all addicts b) Goals should include i) Prevention of relapse of illicit opioid use Medically managed supportive therapy may be needed Nonnarcotic Anti-Inflammatory Analgesics Introduction: Analgesic- pain relieving drug 2 types: 1) Opioid (morphine) *All are considered NSAIDs (nonsteroidal 2) Peripheral acting (aspirin) analgesic, anti-inflammatory agents) Both analgesic/anti-inflammatory Prostaglandins- hormones that produce inflammation Cyclooxygenase- converts precursor to prostaglandin Inhibited by NSAIDs 2 types: 1) Cox-1- prostaglandin that protects/regulates cells in GI tract and in blood platelets during normal conditions 2) Cox-2- respond to stressors, such as inflammation Newer drugs (Celebrex, Vioxx) are selective Cox-2 inhibitors; they don’t affect the GI tract and platelet function. Analgesic effects: Reduce inflammation, body temperature, pain, and act as anticoagulant Nonselective Cyclooxygenase Inhibitors: 1. Aspirin: Prototype NSAID (reduce inflammation and arthritic pains) Most popular form (10-20,000 tons yearly) Inhibits both Cox-1 and 2 (nonselective) Good for low intensity pain Ceiling effect- point at which no more of a drug is effective (effect is maximal) app. 650-1300mg Antipyretic (fever reducing)- inhibits prostaglandins in hypothalamus (body regulation structure) Not to be used with children; Reye’s syndrome (liver/brain damage) Binds to platelets, inhibits functions, reduce blood clotting (heart attack and stroke prevention) Increased oxygen consumption/ carbon dioxide production (panting effect if overdose) Side effects- gastric upset, heartburn, ulcers, bleeding. While intoxicated- ringing in ears, mental confusion, vision difficulties, thirst, hypervent. Those who are aspirin sensitive can experience asthma 2. Acetaminophen: Tylenol- alternative to aspirins; doesn’t possess adverse effects Not useful for arthritic pain/ chronic inflammation Does not inhibit platelets; not good for prevention of stroke or heart attacks Safe for use with children; overdose can cause liver damage Less gastric upset/ringing in ears 3. Ibuprofen: Motrin, Advil, Nuprin, Orudis, etc May be more effective than aspirin, codeine, acetaminophen Lower severity of side effects; however gastric distress and peptic ulcers have been reported Interfere with blood clotting (like aspirin); not recommended for pregnant women Treatment of arthritis, tendonitis, bursitis, dysmenorrheal (menstrual cramping) 4. Phenylbutazone: 1949-used for rheumatoid arthritis; serious side effects Side effects: gastric distress, skin rashes, ulcers, allergies, renal and liver dysfunction Half-life- 2 days 5. Indomethacin, Sulindac, Etodolac: 1963-arthritic pain; 50% experience side effects Side effects: gastric dysfunction, headache Sulindac: inactive, but metabolite (sulindac sulfide) is active; lower reports of gastrointestinal toxicities Etodolac: fewer side effects as well 1st injectable form of NSAID for postsurgical pain Potency of morphine in lower doses; can be used with morphine to reduce dosage by 50% Not recommended for those who are pregnant Side effects: excessive bleeding, renal failure (limited use; 24-48) 6. Keteroloc: 7. Bromfenac: 1997-short-term pain treatment Hepatotoxicity- causes liver toxicity if used more than 10 days 1999-banned from sale Selective COX-2 Inibitors: 1. Celecoxib (Celebrex): 1998- first COX-2 inhibitor; as effective as aspirin No gastrointestinal toxicity/platelet blockage Efficacious against skin and colon cancers Inhibits COX-2 which is overabundant in malignant tumors UV rays induce COX-2 enzymes, NSAIDs decrease tumorigenic effects Peaks in 3hrs; half-life = 11hrs Side effects: abdominal pain, diarrhea, gastric upset 2. Rofecoxib: Treatment of osteoarthritis, acute pain, menstrual cramps Peaks in 3hrs; half-life = 17hrs Antipyretic effect (found to reduce fevers as well) Side effects: nausea, diarrhea, abdominal pain, dyspepsia Nitroaspirins- new class that releases both aspirin and nitric oxide into the body; not available yet, under scrutiny. References: http://cancerweb.ncl.ac.uk/omd/ http://www.centef.ch/pain/Opioids_endog_ligands_dynorphins_link.htm http://chemistry.umeche.maine.edu/CHY132/Enk-Stat.html http://www.wsu.edu:8080/~dee/CHING/OPIUM.HTM