Circadian Rhythm Study
advertisement

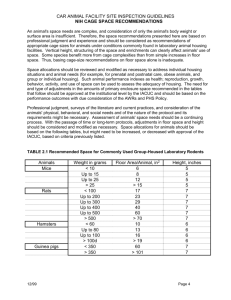
Circadian Rhythm Study Animal Brains: *Store in properly labeled boxes in the designated -80 freezer. Brains must stay frozen! Plasma Collection: 1) Spin kill tubes in cold room centrifuge (Across the hall from lab, Rm. 1503). Centrifuge set to spin at 14G for 5 min. 2) Carefully place tubes back into ice, proceed to lab for pipetting. 3) Four aliquots of plasma will be collected PER animal. Two tubes to be used for CORT assay, two for ACTH assay. For CORT tubes, pipette at least 50l. For ACTH, pipette at least 100l. Excess can go into ACTH tubes. *Two tubes have 50 l for CORT assay *Two tubes have 100 l for ACTH assay 4) Quick freeze plasma on dry ice, store in labeled carrier. 5) Discard waste in biohazard bag, store in Beer Cooler. MATERIALS AND METHODS Animals Female Sprague-Dawley and male Long Evans rats were mated (purchased from Charles Rivers Breeding Laboratories). All animals were housed under constant temperature and light conditions (12hr cycle Lights ON:0500, Lights OFF: 1700) and given food and water ad libitum. All animals were housed in same-sex pairs for two weeks prior to mating. During this time, females underwent vaginal lavage daily to determine their estrous cycle and receptivity to the male. Mating and Birth Procedures Animals were mated following a trio-mating protocol (one male, two females). Identification of sperm from vaginal lavage confirmed the conception day. Once conception was confirmed, the females were housed two to a cage. Two to three days before the expected birth date, the females were separated into individual housing cages. Housing Conditions Within 18 hours of birth, litters were culled to six males, six females. Litters were randomly assigned to one of the following groups: 1) ISO 16- early wean at postnatal day 16 (PND16), Isolated Housing, or 2) GH 16- early wean at postnatal day 16 (PND16), Grouped Housing (3 per cage), or 3) ISO 21- standard wean at day 21 (PND 21), Isolated Housing, or 4) GH 21-standard wean at day 21 (PND 21), Grouped Housing. In addition to being singly housed, the ISO groups were unable to see, touch, or be touched by other animals following the protocol of Garzon and del Rio, 1981. This was achieved by placing white cardboard pieces as dividers between cages and a white paper sheet on the front of the cage, which inhibited visual contact with animals housed in shelves across the room. At weaning, males were separated from the mother and female siblings and housed according to group assignment. All animals remained in the same housing unit as litters which had yet to be weaned until sacrifice. Water and regular vitamin-enriched chow was provided ad libitum. However, earlyweaned animals (PND 16) were given ground chow daily and cow's milk until mature incisors had developed (PND 21). To minimize the effects of handling, animals were left untouched except during culling, weaning, weekly cage changing, height and weight measurement, and during testing. Subgroups Within each litter (n=35; 6 pups per litter), pups were assigned a testing measure: Circadian (C) or Restraint (R). Circadian- Circadian (C) animals were sacrificed over a 24-hour period at time points of 0400 h, 1000 h, 1600 h, and 2000 h and 0000h at the age of PND 69-75. Samples were taken via decapitation of one animal per group per time point. C animals were carried in their cage to the procedure room for sacrifice. Littermates of the Circadian GH animals were brought in the cage as well and set outside the procedure room. One animal was removed from the cage and the remaining animals were returned to the housing room. All C samples were taken within 2 minutes of each animal, and a total of no more than 10 minutes were used to collect samples for all animals. Restraint- Restraint (R) animals were restrained at the age 69-75 three hours after lights off (2000 h). Animals were moved in their cage to a separate testing room. Littermates of R GH animals remained in the cage with the R animal at all times. Restraint was performed for 15 minutes by confining the rat in a small plastic tube so that it allowed minimal movement. During the restraint time, animals were placed in their home cage in the plastic tube. Blood plasma samples were collected from the tail vein at the end of the 15 minute restraint. Phase 1: Animals were returned to their home cage and samples from the tail vein were collected at the end of the restraint (0’ time point). Tail vein samples were also taken 15 and 30 minutes post restraint and then decapitated at 90 minutes to ascertain a complete corticosterone response to stress. There was a maximum of two minutes between each animal. Phase 2: (Due to inconsistencies and difficulties during tail nics in phase 1), a tail vein sample was obtained immediately after the 15 minute restraint. Animals were then returned to their home cage and decapitated 15 minutes post restraint to obtain . Food Consumption and Weight and Length measurement (Phase 2 only) Starting at day 28 continuing every week until sacrifice, food pellets were weighed for each cage by measuring a given amount and subtracting the amount eaten at the end of the week. For GH cages, weight of food eaten was divided by three to estimate the food consumed by each animal. Animals were weighed every two weeks, starting at day 28, until day 67. Animals were measured for length from the tip of the snout to the end of the main body (base of the tail) at day 61. Phase 1 consisted of weight and length measurement on day 70 only Hormone Assesment Stress Response A minimum of 250 l of blood was taken from the tail vein at each timepoint post 15 minute restraint. Samples were placed on ice and later centrifuged at 4C for 5 minutes at 1500 rpm. Plasma was collected and stored in a -80C freezer until the RIA assays were performed. Circadian Rhythm Animals were decapitated using a standard guillotine. Trunk blood was collected in a K3 EDTA vacutainer tube and immediately placed on ice. Samples were later centrifuged at 4°C for 5 minutes at 1500 rpm. Plasma was extracted and stored at -80°C until RIA assays were performed. Corticosterone RIA assay Plasma corticosterone levels were obtained using the Immuchem Double Antibody Corticosterone RIA kit, using 125 I radiolabeling (ICN Biomedicals, Inc., Costa Mesa, CA). Within each separate assay, all time points for all groups were represented. The assay was performed as follows: 10 l of plasma sample was diluted with phosphosaline gelatin buffer (pH 7 + 0.1), containing rabbit gamma globulins, to a dilution of 1:200. 200 l of Corticosterone-3-carboxymethyloxime was added to 100 l of plasma dilution. This antiserum was used to bind 50-60% of the corticosterone 125 I derivative in the absence of nonradioactive corticosterone. 200 l of corticosterone 125 I derivative was added to the solution. The radioactive derivative provided approximately 50,000 cpm at 75% counter efficiency. After incubation, 500 l of precipitant solution (PEG and Goat anti-rabbit gamma globulins contained in TRIS buffer) was added to the solution, immediately precipitating all the antibody bound antigen. The pellet remaining after aspiration was immediately placed in a gamma counter and compared to counts obtained with the standard curve derived from the kit’s corticosterone calibrators. ACTH RIA assay Plasma ACTH levels were obtained using a RSL 125 I hACTH radiolabeling kit (ICN Biomedicals, Inc., Costa Mesa, CA). Within each separate assay, all time points for all groups were represented. The assay was performed as follows: A purified porcine ACTH-conjugates was used to generate an antiserum in rabbits.100 l of the reconstituted antiserum mixture (binding at 20-40% of hACTH 125 I in the presence of nonradioactive hACTH) was added to 100 l of plasma sample. 100 l hACTH 125 I was then added to the antiserum and plasma solution. This radioactive material contains less than 0.4 C per vial. 0.1 ml of this radioactive material will provide 10,000-12,000 cpm at 75% counter efficiency on the iodination date after reconstitution. After a 17-18 hour incubation, 500 l of precipitant solution (PEG and Goat anti-rabbit gamma globulins contained in a 0.01 M phosphosaline buffer pH 7.6) was added to the solution, immediately precipitating all the antibody bound antigen. The pellet remaining after aspiration was immediately placed in a gamma counter and compared to counts obtained with the standard curve derived from the kit’s synthethic ACTH 1-39 calibrators. Behavior Test In order to ascertain the possibility of hyperactive behavior, all animals were introduced to novelty exploration for 60 minutes 5-10 days prior to sacrifice. Locomotion testing took place between the hours of 0900 h-1200 h. Animals were brought in their cage to the testing room, where they were placed one at a time in a clear plastic compartment on a rack holding 18 cages. Six photo sensors recorded lateral and rearing movement of the animal for 60 minutes. Data was recorded electronically, capturing movement in 5minute intervals. During the test, the animals were without food or water. Upon completion of the test, animals were removed individually and placed back into their cages before being returned to their housing room. Statistical Analysis Analysis of Variance (ANOVA) for repeated measures was used for all testing measures except Stress Response results. Variables included between group (ISO16, ISO21, GH16, GH21) differences, housing condition (ISO, GH) and day of weaning (day 16, day 21). Factorial analysis was also computed for all testing measure to ascertain differences between all variables for each time point. When the level of significance reached p<0.05 post-hoc analyses were performed using Fisher’s PLSD test.