Supplementary Information (doc 456K)
advertisement

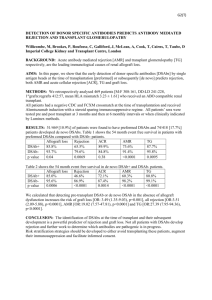
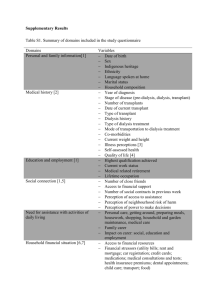
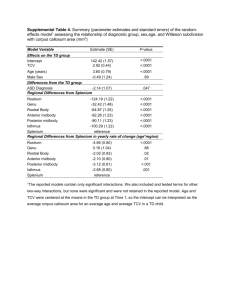
Supplementary Table 1. Pre-treatment variables. N Age (y) (median [range]) Male N (%) CP duration mo (median [range]) Prior hydroxyurea >1 mo N (%) Prior interferon-α N (%) Splenomegaly N (%) WBC X10E9/L (median [range]) Hemoglobin g/L (median [range]) Platelets x10E9/L (median [range]) Blood blasts % (median [range]) Blood basophils % (median [range]) Bone marrow blasts % (median [range]) Sokal score N (%) Low-risk Intermediate-risk High-risk Unknown Median follow-up survivors mo (range) Imatinib 172 38 (14–55) 125 (73) 1 (0–11) 44 (26) 14 (8) 62 (36) 18.1 (3.2–533) 123 (55–172) 298 (45–1904) 0 (0–4) 4 (0–18) 0.5 (0–10) Transplant 176 37 (12–53) 136 (77) 5 (0–11) 161 (92) 36 (21) 31 (18) 6.6 (1–45) 130 (68–182) 222 (16–1936) 0 (0–7) 3 (0–19) 1.5 (0–8) 0.077 0.323 <0.0001 <0.001 0.001 <0.0001 <0.0001 0.018 <0.0001 0.045 <0.0001 0.509 102 (59) 44 (26) 12 (7) 14 (8) 49 (12–143) 108 (61) 32 (18) 10 (6) 26 (15) 82 (32–137) 0.694 0.095 0.620 0.052 <0.0001 CP, chronic phase. 1 P-value Supplementary Table 2. Relative risks of event (1-EFS), treatment-failure (1-PFS) and death (1-survival). (Left-truncation method)* N RR (95% CI) P-value 1-EFS Main effect Imatinib 172 1.0 Transplant 176 3.6 (2.0–6.8) <0.0001 Other Blood basophils < 5 228 1.0 ≥5 120 2.2 (1.3– 3.9) 0.0043 Blood blasts No 300 1.00 Yes 48 2.2 (1.1–4.3) 0.0232 1-PFS Main effect Imatinib 172 1.0 Transplant 176 5.3 (2.4–11.7) <0.0001 Other Blood basophils < 5 228 1.0 ≥5 120 2.3 (1.2–4.3) 0.0109 Death Main effect Imatinib 172 1.0 Transplant 176 42 (6–309) 0.0003 Other Blood basophils < 5 228 1.0 ≥5 120 2.2 (1.1–4.4) 0.0281 * Left-truncated Cox proportional hazards regression models were used to evaluate the relative risk of subjects receiving imatinib versus a transplant. Variables considered in the multivariate models were: age (≥40 versus <40 years); subject gender (male versus female); splenomegaly (yes versus no); WBC (≥30 vs <30×10E9/L); hemoglobin concentration (<120 versus ≥120 g/L); platelet level (<450 versus ≥450×10E9/L); blood blasts (no versus yes); blood basophils (<5% versus ≥5%); bone marrow blasts (<5% versus ≥5%; and Sokal score (unknown versus 1 versus 2 versus 3; 25). The assumption of proportional hazards for each variable in the Cox model was tested using time-dependent covariates. Figures and tests showed there is no statistical evidence proportionality assumptions did not hold. 2 A backward stepwise model selection approach was used to identify all significant risk factors. Each step of model building contained the main effect for imatinib versus transplant and risk factors significant at a P≤0.05 level were retained in the final model. Potential interactions between main effect (imatinib versus transplant) and all significant risk factors were tested; no interaction was detected. Adjusted probabilities of EFS, PFS and survival were generated from the final Cox models stratified on treatment with imatinib or transplant, and weighted averages of covariate values using the pooled sample proportion as the weight function. These adjusted probabilities estimate likelihood of outcomes in populations with similar prognostic factors. RR, relative risk; EFS, event-free survival; 3 PFS, progression-free survival. Supplementary Table 3. (Not-left-truncation method) Outcome: EFS: 2 Year 4 Year 6 Year 8 Year 10 Year PFS: 2 Year 4 Year 6 Year 8 Year 10 Year OS: 2 Year 4 Year 6 Year 8 Year 10 Year Adjusted probabilities of EFS, PFS and survival Imatinib At Risk P (95% CI) 153 93 (88 – 96)% 81 92 (87 – 95)% 33 92 (87 – 95)% 12 92 (87 – 95)% 8 92 (87 – 95)% 157 96 (92 – 98)% 86 96 (91 – 98)% 37 96 (91 – 98)% 15 96 (91 – 98)% 11 96 (91 – 98)% 158 99 (96 – 100)% 87 99 (96 – 100)% 37 99 (96 – 100)% 15 99 (96 – 100)% 11 99 (96 – 100)% EFS, event-free survival; Transplant At Risk P (95% CI) 144 79 (72 – 84)% 122 77 (70 – 83)% 93 76 (69 – 82)% 38 75 (67 – 81)% 17 75 (67 – 81)% 151 84 (77 – 89)% 126 81 (74 – 86)% 95 80 (73 – 85)% 39 78 (70 – 84)% 17 78 (70 – 84)% 151 84 (77 – 89)% 127 81 (74 – 87)% 96 81 (73 – 86)% 40 79 (71 – 85)% 18 79 (71 – 85)% PFS, progression-free survival. 4 P 0.0003 0.0002 0.0001 0.0001 0.0001 0.0001 <0.0001 <0.0001 <0.0001 <0.0001 <0.0001 <0.0001 <0.0001 <0.0001 <0.0001 Supplementary Table 4. Relative risks of event (1-EFS), treatment-failure (1-PFS) and death (1-survival). (Not-Left-truncation method) Outcome event 1-EFS: Main Effect: Imatinib Transplant Other Risk factor: Blood basophiles < 5 ≥5 Blood blast No Yes 1-PFS: Main Effect: Imatinib Transplant Other Risk factor: Blood basophiles < 5 ≥5 Overall Mortality: Main Effect: Imatinib Transplant Other Risk factor: Blood basophiles < 5 ≥5 RR, relative risk; N RR (95% CI) P 172 176 1.00 3.09 (1.68 – 5.69) 0.0003 228 120 300 48 1.00 2.25 (1.30 – 3.90) 1.00 2.16 (1.10 – 4.23) 172 176 1.00 4.91 (2.23 – 10.83) <0.0001 228 120 1.00 2.31 (1.23 – 4.33) 0.0095 172 176 1.00 37.86 (5.13 – 279) 0.0004 228 120 1.00 2.23 (1.10 – 4.49) 0.0256 EFS, event-free survival; 5 0.0040 0.0254 PFS, progression-free survival. Supplementary Table 5. Left-truncated analysis results (truncated at time of imatinib or transplant) N RR (95% CI) P Outcome event 1-PFS: Main Effect: Imatinib Transplant Other Risk factor: Blood basophiles < 5 ≥5 1-EFS: Main Effect: Imatinib Transplant Other Risk factor: Blood basophiles < 5 ≥5 Blood blast No Yes Overall Mortality: Main Effect: Imatinib Transplant Other Risk factor: Blood basophiles < 5 ≥5 RR, relative risk; 172 176 1.00 5.38 (2.44 – 11.88) <0.0001 228 120 1.00 2.27 (1.21 – 4.28) 0.0108 172 176 1.00 3.61 (1.94 – 6.71) <0.0001 228 120 300 48 1.00 2.23 (1.29 – 3.88) 1.00 2.20 (1.12 – 4.32) 172 176 1.00 14.47 (4.37 – 47.96) <0.0001 228 120 1.00 2.41 (1.23 – 4.75) 0.0109 EFS, event-free survival; 6 0.0044 0.0226 PFS, progression-free survival. Supplementary Table 6. Outcome event 1-PFS: Main Effect: Imatinib Transplant Other Risk factor: Blood basophiles < 5 ≥5 1-EFS: Main Effect: Imatinib Transplant Other Risk factor: Blood basophiles < 5 ≥5 Blood blast No Yes Overall Mortality: Main Effect: Imatinib Transplant Other Risk factor: Blood basophiles < 5 ≥5 RR, relative risk; Not-Left-truncated analysis results (truncated at time of imatinib or transplant) N RR (95% CI) P 172 176 1.00 4.99 (2.26 – 11.00) <0.0001 228 120 1.00 2.31 (1.23 – 4.33) 0.0095 172 176 1.00 3.09 (1.68 – 5.69) 0.0003 228 120 300 48 1.00 2.24 (1.29 – 3.89) 1.00 2.16 (1.10 – 4.24) 172 176 1.00 13.21 (3.99 – 43.71) <0.0001 228 120 1.00 2.44 (1.24 – 4.81) 0.0097 EFS, event-free survival; 7 0.0041 0.0248 PFS, progression-free survival. Supplementary Figure 1. CONSORT diagram. TRM, treatment-related mortality; TKI, tyrosine kinase inhibitor; NR: no response; CHR, complete hematological response. 8 Supplementary Figure 2. Adjusted probabilities of EFS, PFS and survival from diagnosis (Not-Left-truncation method). EFS, event-free survival; A: EFS; B: PFS; PFS, progression-free survival. 9 C: Survival.