Thermodynamics Chapter Three Lecture: Noor M. Jasim
advertisement

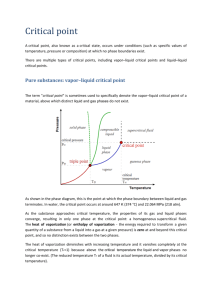
Thermodynamics Chapter Three Lecture: Noor M. Jasim THERMODYNAMICS Chapter Three PROPERTIES OF A PURE SUBSTANCES Noor M. Jasim 2009 Thermodynamics Chapter Three Lecture: Noor M. Jasim Chapter Three Properties of a pure substances 3.1 PURE SUBSTANCE ■ A substance that has a fixed chemical composition throughout is called a pure substance. Water, nitrogen, helium, and carbon dioxide, for example, are all pure substances. A pure substance does not have to be of a single chemical element or compound, however. A mixture of various chemical elements or compounds also qualifies as a pure substance as long as the mixture is homogeneous. Air, for example, is a mixture of several gases, but it is often considered to be a pure substance because it has a uniform chemical composition. A mixture of two or more phases of a pure substance is still a pure substance as long as the chemical composition of all phases is the same. 3.2 PHASES OF A PURE SUBSTANCE ■ there are three principal phases—solid, liquid, and gas—a substance may have several phases within a principal phase, each with a different molecular structure. Carbon, for example, may exist as graphite or diamond in the solid phase. 3–3 PHASE-CHANGE PROCESSES OF PURE SUBSTANCES ■ 1. Compressed liquid and saturated liquid consider a piston- cylinder device containing liquid water at 20Coand 1atm pressure (state 1). Under these conditions, water exists in the liquid phase and it is called a compressed liquid or a sub cooled liquid. heat is now added to txe water until its rises to 100Co (state 2). At this point water still, liquid, but any heat addition will cause some of liquid to of liquid to vaporize. The state 2 is called a saturated liquid. 2. Saturated vapor and superheated vapor. Once boiling starts, the temperature will stop rising until the liquid is completely vaporized. During a vaporization process, the temperature will remain constant. Midway about the vaporization line (state3 )the cylinder contains equal amounts of liquid and vapor. As we continue adding heat, the vaporization process will continue until the last drop of liquid is vaporized (state 4). State 4 is called a saturated vapor state. Any further heat transfer will result in an increase in both the temperature and the specific volume (state 5). A vapor that is not about to condense is called a superheated vapor. The constant pressure phase- change process described as shown in T-V diagram. 3–4 Property Diagrams for phase- changes processes : ■ Thermodynamics Chapter Three Lecture: Noor M. Jasim Saturation Temperature (T sat): The temperature at which a pure substance starts boiling at a given pressure. Saturation pressure (p sat ): The pressure at which a pure substance starts boiling at a given temperature. Critical point: The point at which the saturated liquid and Saturated vapor states are identical. 1. The T-V Diagram: 2. P-V Diagram: Thermodynamics Chapter Three Lecture: Noor M. Jasim 3. P-T Diagram (phase diagram ) 3–5 Enthalpy: ■ The property that which the combination of properties U+PV, and it is the heat contents or the total heat Therefore; H=U+P.V h= u+ p.v (kj) (kj/kg) 3–3 Property Tables ■ 1. Saturated liquid and saturated vapor states:The subscript (f) is used to denote properties of saturated liquid, and the subscript (g) to denote the properties of saturated vapor. Another subscript used is (fg), which denotes the difference between the saturated vapor and saturated liquid values of the same property. For example:vf =specific volume of saturated liquid vg =specific volume of saturated vapor vgf =Difference between vf and vg (vfg= vg- vf) but; hgf =The enthalpy of vaporization (or latent heat of vaporization). Thermodynamics Chapter Three Lecture: Noor M. Jasim Example 1// A rigid tank contains 50kg of saturated liquid water at 90Co .Determine the pressure in the tank and the volume of the tank. Solution // Since the saturation conditions exist in the tank the pressure must be the saturation pressure at 90Co P=70.14 kpa from sat steam tables The specific volume of the saturated liquid at 90oC is vf = 0.001036m3/kg then the total volume of the tank is determined from V= m. vf =(50 kg).(0. 001036m3/kg)= 0.0518 m3 Example 2 // A mass of 200g of saturated liquid water is completely vaporized at a constant pressure of 100kpa, determine : (a-)The volume change (b-)The amount of energy added to the water. Solution // V1= m. vf =(0.2 kg).(0. 001043m3/kg) V2= m. vg=(0.2 kg).(1.694m3/kg) a- The amount change per unit mass during a vaporization process is vfg at 100 kpa vfg= vg - vf = 1.694 -0.001043 m3/kg. thus AV= m. vfg =(0.2 kg).(1.694m3/kg)=0.3386 m3 b- The amount of energy needed to vaporize the unit mass of a substance at a given pressure is the enthalpy of vaporization at that pressure which at 100kpa :hfg=2258 kj/kg Thus the amount of energy added is m*hfg=0.2*2258=451.6 kj Thermodynamics Chapter Three Lecture: Noor M. Jasim 2-Saturated Liquid–Vapor Mixture During a vaporization process, a substance exists as part liquid and part vapor. This is done by defining a new property called the quality x as the ratio of the mass of vapor to the total mass of the mixture: The total volume V is the sum of the two: νx=νf + x*νfg The same form of expression holds for other specific properties:- ux=uf + x*ufg hx=hf + x*hfg Let Y=any property for substance (ν ,h ,u ) then,the The values of the average properties of the mixtures are always between the values of the saturated liquid and the saturated vapor properties: Yf ˂ Y ˃ Yg ,then the substance is mixture. Yf ˃ Y ,then the substance is compressed liquid. Yg ˂ Y ,then the substance is superheated vapor. Thermodynamics Example 3 Chapter Three Lecture: Noor M. Jasim Thermodynamics Chapter Three Lecture: Noor M. Jasim Example 4 Example 5: Water at 200 kPa with a quality of 25% has its temperature raised 20oC in a constant pressure process. What is the new quality and volume? Solution: State 1 from Table B.1.2 at 200 kPa v = vf + x vfg = 0.001061 + 0.25 × 0.88467 = 0.22223 m3/kg State 2 has same P from Table B.1.2 at 200 kPa T2 = Tsat + 20 = 120.23 + 20 = 140.23oC so state is superheated vapor x = undefined v = 0.88573 + (0.95964 – 0.88573)20150 - 120.23 = 0.9354 m3/kg Thermodynamics Chapter Three Lecture: Noor M. Jasim 3- Superheated Vapor In the region to the right of the saturated vapor line and at temperatures above the critical point temperature, a substance exists as superheated vapor. Since the superheated region is a single-phase region (vapor phase only), temperature and pressure are no longer dependent properties and they can conveniently be used as the two independent properties in the tables. Example 6 4- Compressed Liquid Compressed liquid tables are not as commonly available, and Table A–7 is the only compressed liquid table. The format of Table A–7 is very much like the format of the superheated vapor tables. One reason for the lack of compressed liquid data is the relative independence of compressed liquid properties from pressure. Variation of properties of compressed liquid with pressure is very mild. In the absence of compressed liquid data, a general approximation is to treat compressed liquid as saturated liquid at the given temperature. This is because the compressed liquid properties depend on temperature much more strongly than they do on pressure. Thus, ν ≈ νf at Tsat u ≈ uf at Tsat Although the above approximation results in negligible error in v and u, the error in h may reach undesirable levels. However, the error in h at low to moderate pressures and temperatures can be reduced significantly by evaluating it from: h ≈ hf + νf ( P-Psat) Thermodynamics Example 7 Chapter Three Lecture: Noor M. Jasim Thermodynamics Chapter Three Lecture: Noor M. Jasim 3–7 linear interpolation ■ The states encountered when solving problems often do not fall exactly on the grid of values provided by property tables. Interpolation between adjacent table entries then becomes necessary. Care always must be exercised when interpolating table values. The tables provided in the Appendix are extracted from more extensive tables that are set up so that linear interpolation . Example 8:Find the pressure and temperature for saturated vapor R-12 with v = 0.1 m3/kg. Solution: Table B.3.1 Look at the saturated vapor column vg and it is found between −20°C and −15°C. We must then do a linear interpolation between these values To understand the interpolation equation look at the smaller and larger triangles formed in the figure. The ratio of the side of the small triangle in v as (0.10885 - 0.1) to the side of the large triangle (0.10885 - 0.09101) is equal to 0.4961. This fraction of the total ΔP = 182.6 - 150.9 or ΔT = -15 -(-20) is added to the lower value to get the desired interpolated result.