Molar Volume of Carbon Dioxide
advertisement

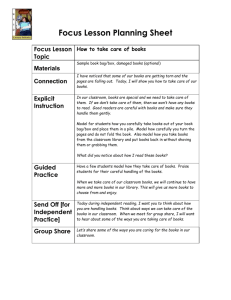
Molar Volume of Carbon Dioxide Introduction: Gases occupy a certain volume of space which depends on 1) temperature, 2) pressure and 3) amount or number of moles of gas in the sample. Purpose:This laboratory experience is designed to help you determine the volume of 1 mole of a gas at Standard Temperature and Pressure. The gas we will use (CO2) is conveniently provided by reaction of the popular cold remedy Alka-Seltzer. The following reaction occurs as citric acid reacts with sodium hydrogen carbonate (baking soda). Check the product box to find out exactly how much citric acid and sodium bicarbonate are contained in each product tablet. What are the scientific principals involved in this lab? (There are a lot!) The reaction is as follows: Hypothesis: Based on what you already know about gases at STP, what is your hypothesis about the molar volume of CO2? _____________________L/mol C3H5O(COOH)3(aq) + 3 NaHCO3(aq) C3H5O(COONa)3(aq) + 3 H2O(l) + 3 CO2(g). Citric acid Sodium hydrogen carbonate Materials: Alka-Seltzer tablet Water Snack size Ziploc bag. Thin stem micropipette (with most of the stem cut off) Short (5-8 cm) section of a soda straw Celsius thermometer 100 mL graduated cylinder Sink full of room temperature water Method/Procedure: 1. Safety goggles are NOT necessary for this laboratory. 2. Measure and record the mass of one whole Alka-Seltzer tablet. 3. Break the tablet into 4 approximately equal pieces, and record the mass of ¼ of a tablet to use in your experiment. 4. Share the remaining pieces and the mass of the whole tablet with 3 other lab groups. 5. Place no more than ¼ of a tablet into one corner of the Ziploc bag. 6. Fill the micropipette completely with tap water and place it in the opposite corner of the bag. Be careful not to get the ¼ tablet wet!! 7. Seal the bag most of the way and attempt to remove all excess air from the bag. This may be accomplished by placing the end of the soda straw into the opening of the bag and using your mouth to suck out the remaining air in the bag. 8. Remove the straw and seal the bag completely. 9. Squeeze the micropipette and allow the water to contact the tablet piece. The resulting reaction will fill the bag with CO2 gas. 10. Read and record the temperature of the water in the sink using the thermometer. 11. Your instructor will provide the current barometric pressure on the board in the classroom. Record this value also. 12. When the reaction of the tablet and water is complete, the gas will be collected by water displacement. 13. Fill the 100 mL graduated cylinder with water from the sink and invert it being careful to keep the open mouth of the cylinder below the water level. 14. Push the plastic bag into the water in the sink and put one corner of the bag into the open end of the graduated cylinder. (See diagram below.) 15. Carefully puncture or cut the corner of the bag with a pencil or scissors. 16. Gently squeeze the gas out of the plastic bag collecting the bubbles in the graduated cylinder. Be careful not to let any of the gas bubbles escape. This can prove to be a major source of error. 17. Once the gas has all been collected, move the graduated cylinder up or down in the sink until the water level inside the tube is even with the water level in the sink. This insures that the pressure of the gases inside the cylinder is the same as the pressure of the atmosphere in the room. Record the volume of the gas in the cylinder. 18. Clean up your experiment. Return the micropipette, but dispose of the plastic bag and soda straw. Collected CO2 gas plus water vapor. Graduated cylinder filled with water and inverted. Cut or puncture corner of bag. Be careful not to let any gas escape. Sink Ziploc bag filled with CO2 gas. Water Thermometer Data: Mass of whole tablet (g) Mass of ~ ¼ of a tablet (g) Mass citric acid in 1 tablet (g) Mass sodium hydrogen carbonate in 1 tablet (g) Barometric Pressure (mm Hg) Temp. of water in sink (C) Volume of gas in cylinder (mL) (V1) Partial pressure of water vapor at temperature of water in sink. Additional information: Molar mass of citric acid Molar mass of sodium hydrogen carbonate Combined Gas Law Dalton’s Law of Partial Pressures 192.12 g/mol 84.01 g/mol P1V1 = P2V2 T1 T2 P T = P1 + P2 + … Calculations: Show all calculations clearly. Calculating gas volume at STP and corrected for water vapor contamination. 1. Correct temperature to Kelvins: T1 = _____________K. 2. Correct the gas pressure for the presence of water vapor: PCO2 = Pbaro – PH2O P1 = ______________mmHg. 3. T2 (standard temperature) = ________________K. 4. P2 (standard pressure) = ________________mmHg. 5. Calculate V2 at STP using the combined gas law ________________mL. 6. Convert V2 to liters ________________L. Calculating moles of gas from the balanced equation and initial reactant masses. 1. Using the information from the Alka-Seltzer package and the balanced equation, determine with stoichiometry which reactant (citric acid or sodium bicarbonate) is the limiting reagent. 2. Calculate the mass of limiting reagent in the piece of tablet you used. 3. Convert the mass of limiting reagent in your piece of tablet to moles. 4. Use the balanced equation to calculate the number of moles of CO2 produced: n = _____mol Calculating Molar Volume of the Gas 1. Calculate the experimental molar volume of CO2 gas (V2/n). Molar V = _________L/mol. 2. Calculate your % yield based on the known molar volume of gases at STP. 3. Calculate your % error. Conclusion: (Use third person passive voice- no personal pronouns.) 1. 2. 3. 4. 5. What can be concluded about the experimental molar volume of a gas? Is hypothesis accepted or rejected? What did the experimenter learn? What are the applications of the principles of this lab? What were the major sources of error?