World Journal of Engineering INCREASING THE OCTANE
advertisement

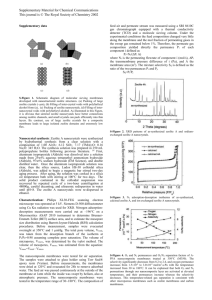
World Journal of Engineering INCREASING THE OCTANE NUMBER OF GASOLINE USING PRASEODYMIUM(III)ETHYLENE DIAMINE TETRA ACETATE/ACTIVATED CLINOPTILOLITE ZEOLITE Dewi Tristantini*, Eny Kusrini, and Vino Hasyim Chemical Engineering Department, Faculty of Engineering, University of Indonesia, Depok 16424, Indonesia Introduction Lanthanides (rare earth) in a form of oxides and a complex used as a catalyst since a few years ago. For example, it was used in oxidation and/or combustion reaction, isomerization, dehydrogenation, dehydration, control of sulfur oxidation, carbon oxides metanation, DielsAlder reaction etc. [Kilbourn, 1986]. Gasoline octane number can be increased by adding the TEL (Tetra Ethyl Lead) but after some time used, the researchers found that TEL weakness can lead to fuel emissions that endanger human health. Many studies were conducted to find materials that able to raise octane number of gasoline as a substitute for TEL. One of active agent to subtitute TEL is Praseodymium. Praseodymium (Pr-III) is a member of lanthanide. Properties of lanthanide complexes can be studied better when Ln(III) complexes change into a form of lanthanide complex nanoparticles than in a form of large crystal (bulk crystal) [Kusrini et al. , 2009]. The catalyst based complex Praseodymium (Pr) is made by impregnation of large crystal (bulk) Pr(III)EDTA complexes and nanoparticles Pr(III)EDTA respectively into zeolite and then the catalysts were characterized to determine its properties. Fabrication of lanthanide complexes in the form of Gd(III) with EDTA ligands by hydrothermal method has been reported previously [Kusrini et al., 2010]. Clinoptilolite zeolite is one of the catalyst supports that has almost all good support properties required. One of the properties that must be possessed of gasoline is the high octane number of the fuel. In this study we explore a subtance that can increase the octane number of gasoline by creating a large crystal and nanoparticles catalysts Praseodymium (III)EDTA which impregnated into Clinoptilolite zeolite. Then this ingredients will be a gasoline additive to increase the octane number. Experimental Large crystal impregnation of Praseodymium (III)-EDTA complex on Clinoptilolite Zeolite Zeolite is weighed for 4 grams. Zeolite immersed in solution of 2% weight/weigt large crystal Pr-EDTA complex and stired evenly for 1 hour while heated on a hot plate at 60oC. Then the imprenated-zeolite was put into a sentrifuge at 2000 rpm for 10 minute continue with heat it up in an oven at 105oC to remove the excess water. Thr catalyst then calcinated at 400oC for 4 hours. The catalyst of Pr(III)-EDTA/zeolite large crystal then characterized by surface area analyzer, XRD, XRF and FTIR. Impregnation of Pr-EDTA complex nanoparticles on Clinoptilolite Zeolite The same procedure with the previous catalysts was done to make nanoparticles of Pr-EDTA complexes, except the solution is made of PrEDTA complexes solution 2% w/w. Impregnation of Praseodymium (Pr6O11) Oxide on Clinoptilolite Zeolite A similar prfocedure with the two previous catalysts was done to make Praseodymium (Pr6O11) Oxide on Zeolite but the solution is made from 1 gram Praseodymium oxidedissolved into 10 mL HCl 10% solution. Test of Pr/zeolite oxide catalyst activity was done to determine the increasing gasoline octane number. Activity Test of increasing gasoline octane number Balanced 3 grams of each catalyst and put it into 60 ml gasoline respectively then stired for 2 minutes. The precipitates were filtered using whatman filter paper No.41. Filtrat octane numbers were measured by Octane meter SHATOX SX-200. Gasoline obtained from the filtration was analyzed using GC-MS. 1129 World Journal of Engineering Table 1. Gasoline octane number measurement with and without addition of Pr(III)EDTA catalysts Octane Name of Mixture Number Gasoline (pure) 88.2 Gasoline + Zeolit Catalyst 88.8 Gasoline + Large Crystal Catalyst Pr(III)-EDTA/ Zeolit 89.2 Gasoline + Nanoparticle Catalyst Pr(III)EDTA/Zeolit 89.6 Gasoline + Pr Oxide 88.4 Gasoline + Pr/Zeolit Oxide 88.4 Results And Discussions Based on XRF characterization, the Pr content in the Pr(III)-EDTA/Zeolite large crystal catalyst and Pr(III)-EDTA/Zeolite nanoparticles is 0.42% and 0.52% respectively. This result shows that the Pr succesfully impregnated into zeolite, although impregnation efficiency is still low (20-26%). The BET test result shows that most of zeolite pores is less filled by large crystal than Pr(III)EDTA nanoparticles (BET : initially 30.86 m2/g, 24.09 m2/g and 9.91 m2/g respectively catalyst). Based on the results of FTIR analysis of the visible spectrums (Fig. 1.) are almost the same between the zeolite and the impregnated catalyst with Pr (III)-EDTA large crystals or Pr (III)EDTA nanoparticles. This is likely caused by the Pr (III)-EDTA only interact physically with the zeolites without changing the crystal structure of zeolite. Conclusion The impregnation of Praseodymium (III)-EDTA large crystal and Praseodymium (III)-EDTA complex nanoparticles into the Clinoptilolite zeolite are succes. It is proved from the results of the catalyst characterization result by XRF. The result of GC-MS shows an increase of iso oktane component (C8H18) % peak area after addition of zeolite, Pr(III)-EDTA/Zeolite large crystal catalyst and Pr(III)-EDTA/Zeolite nanoparticles catalyst respectively. In addition, it shows a decrease of n-oktana component (C8H18) % peak area when the catalyst of Pr(III)EDTA/Zeolite nanoparticles is added to gasoline. (c) (a) (b) (a) Zeolite (b) Pr(III)-EDTA/Zeolite Nanoparticle (c) Pr(III)-EDTA/Zeolite Large Crystal Fig. 1. FTIR Spectrum of Zeolite Catalyst, Pr(III)-EDTA Large Crystal Catalyst, Pr(III)-EDTA Nanoparticle Catalyst References 1. Chen L., Wang X.*, Guo H., Guo X., Wang Y., Liu H, Li G. 2007. Hydroconversion of n-octane over nanoscale HZSM-5 zeolites promoted by 12-molybdophosphoric acid and Ni. Catalysis Communications 8 ,pp. 416– 423. 2. Kilbourn, B.T. (1986). The Role Of Lantanida. J. Less-Common Metals, 126, pp. 101-106. 3. Kusrini, E., Saleh, M.I. (2009). Luminescence and structural studies of yttrium and heavier lanthanide- picrate complexes with pentaethylene glycol, Inorg. Chem. Acta, 362, 4025. Activity Test of increasing gasoline octane number Table 1 shows the result of catalyst activitiy test in increasing gasoline’s octane number. The activity of catalyst on gasoline is probably caused by a trace conversion of straight chain alkanes to branched chain alkanes. The increasing amount of catalytically converted of straight chain alkanes to branched chain alkanes correspond to the quality of gasoline [Chen et al., 2007]. 1130