Chemical compostion of the samples
advertisement

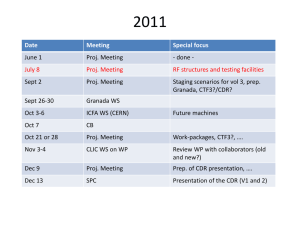
Analysis of as received samples Chemical composition of the samples: We remind that the surface region observed was on the flat part of the couples. Both surfaces CLIC I and CLIC IIa (figure 1)are strongly contaminated by C and some traces of Si, as summarized in table I and comared to the reference (surface of the cut). Sample/ element CLIC I CLIC IIa reference Table I C[%] O[%] Cu[%] Si[%] 66.2 65.7 79.1 23.1 23.1 14.2 1.3 3.5 2.4 9.4 7.8 4.4 The fact that Si is present also on the cut reference face might indicate that part of the Si contamination is generated by the packaging in the plastic bag after cutting. However, this hypothesis can be discarded, since we measured the surface composition of a copper sample, which was stored for 2 hours in a similar plastic bag and we did not find any trace of Si. The Si intensity (peak area) for the CLICI and CLICIIa samples is almost twice as large as on the reference. No clear conclusions can be drawn about the origin of the Si. III I IIa XP SS Figure 1 Chemical species: Both samples have identical Cu2p spectra (worse signal to noise on CLIC I). The Cu 2p peaks (fig 2) indicate the presence of at least two types of Cu within the probing depth of the XPS technique. The main contribution is at 932.4eV and the lower one is around 934.6 eV. From the position of the LMM Auger line at 336.7 eV we can conclude that Cu is mainly present as Cu2O. The minor contribution can be ascribed to Cu(OH)2 : no traces of metallic Cu or CuO are detected. This minor contribution almost vanishes after about 30 minutes irradiation, which is the time needed to acquire the multiplex spectrum, therefore its true intensity is higher than the measured one. The reference sample exhibits only the main peak at 932.8 eV (a shift 0f about 0.4eV with respect to the other samples might be given by adjustment problem due to shape of the sample and is systematic on all lines) and no minor peak. The Cu LMM line has maximum at 336.8eV with a shoulder at 339.6eV, which could not be ascribed to any known Cu feature. We conclude that Cu is present as Cu2O in this case too. The O1s line (fig 3) is similar for all samples and is constituted of at least two components: the main peak is at 532-532.3 eV and the other (about 1/5-1/6 of the total intensity) at 530.5-530.7 eV. The second peak corresponds to the oxygen in Cu2O oxide, whereas the main component is more difficult to interpret. It might be due to water, hydroxides or oxygen containing hydrocarbons. It is not correlated to the Cu 2p peak ascribed to Cu(OH)2 since it does not markedly decrease upon irradiation.. The energy position of the Si 2p line corresponds to silicones and/or silicates (102102.4eV) on all samples. However , the fact that no other impurity elements are detected seems to discard the possibility to have silicates. Cu 2p lines on CLIC IIa Cu2O intensity [a.u.] Cu(OH)2 ? 35000 980 970 960 950 binding energy [eV] figure 2 940 930 920 O1s line of CLIC IIa 35000 contaminants 33000 Intensity [a.u.] 31000 29000 Cu2O 27000 25000 23000 21000 19000 17000 540 538 536 534 532 530 528 526 binding energy [eV] Figure 2 XPS data for CLIC surfaces 90 concentrations [at%] 80 70 60 C[%] O[%] Si[%] Cu[%] 50 40 30 20 10 0 CLIC I CLIC IIa Ref 524