Supplemental Information Using a Biokinetic Model to Quantify and
advertisement

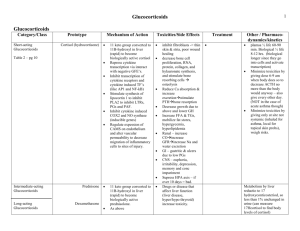
Supplemental Information Using a Biokinetic Model to Quantify and Optimize Cortisol Measurements for Acute and Chronic Environmental Stress Exposure During Pregnancy M Smith1, WC Griffith1, S Beresford2,4, M Vredevoogd1,3, EM Vigoren1,3, EM Faustman1,3 1 Institute for Risk Analysis and Risk Communication, Department of Environmental and Occupational Health Sciences 2Department of Epidemiology and 3Northwest Center for the National Children’s Study, University of Washington, Seattle, WA; 4Cancer Epidemiology, Prevention and Control, Fred Hutchinson Cancer Research Center, Seattle, WA Summary of Contents: This file contains equation S1, showing the corticosteroid binding globulin (CBG) and albumin binding properties of cortisol, Table S1 showing model input and running parameters. The figures show blood protein distribution profiles (Figure S1), construction of diurnal cortisol profiles (Figure S2), partitioning of cortisol between binding proteins (Figure S3), accumulation of cortisol in hair (Figure S4) and diurnal variability of blood cortisol concentrations (Figure S5). Supplementary Equation 1: Cubic Equation for Free Cortisol from Dorin et al 20091: F3+pF2+qF+r=0, Where p=TotA+TotC−TotF+KA+KC, q=TotA×KC+TotC×KA−TotF×(KA+KC)+KAKC, and r=−KAKC×TotF. F is free cortisol, TotA is total albumin, TotC is total CBG, KC is and KA is the mean albumin concentration and KC is the mean CBG concentration. KC and KA distributions used in this model are presented in figure S2. 1 Table S1: Parameters used to construct and validate our biokinetic model. Modeled free cortisol pulse characteristics summarized from parameters in 2. Rate constant parameters are given with relevant references used in their derivation. Use in Model Episodic Stressors Diurnal Variation- baseline decreased by 50% at night Rate Constants Protein Binding Parameters Relevant References Parameter Value Pulse Frequency GM 38 and GSD 13 Pulses/Day 2 Pulse Height GM 80 and GSD 44 nmol/L 2 Interclass Correlation Coefficient Baseline Average 0.37 3 Half-life GM 49 and GSD 42 minutes Metabolism Rate Constant 26.7 hours -1 2,5 Rate constant for Incorporation into Hair 2.96e-5 hours -1 6-9 Rate Constant for Transfer to Saliva 0.00242 hours -1 Rate Constant for Urinary Excretion 0.304 hours -1 2,5 Albumin Concentration GM 710 and GSD 1.05 µmol/L 12 CBG Concentration GM 765 and GSD 1.27 nmol/L 1,13 CBG Dissociation Constant 33nM 1 Albumin Dissociation Constant 137,800 nM 1 Serum Saliva Equilibrium Constant 2.6 14 Saliva per Day 1.5 L 11 Serum Volume 2.7 L 11 Mean Free Blood Cortisol Concentrations 7.12 nmol/L 1,2 Urinary Free Cortisol per Day 1440 nmol 5 Hair Cortisol Concentration 21 pg/mg 9 GM 66 and GSD 50 nmol 2,4 2 10,11 Physiological Parameters Average Cortisol Concentrations *Geometric Mean=GM, Geometric Standard Deviation= GSD, CBG= Corticosteroid Binding Globulin 2 Figure S1: Cortisol binding protein concentrations and variability used in this model. We used means and standard deviations from 13 for CBG concentrations (A-C) and12 for albumin concentrations (D). Using this data we ran 1000 simulations to create the distributions shown above. We used distributions A and D in our model. The geometric mean for the CBG distribution in A is 765 nmol/L with a geometric standard deviation of 1.27 nmols/L. The geometric mean and standard deviation for the albumin distribution in D is 710µmol/L with a geometric standard deviation of 1.05 µmol/L. During pregnancy, CBG concentrations incrementally increase to approximately 1950nmol/L by the end of the third trimester. 3 Figure S2: Construction of a daily blood cortisol profile showing first baseline diurnal variation, then pulsatile cortisol secretion and finally a combination of the two, reflecting a complete blood cortisol profile for one day. Because pulsatile secretion varies by individual and by day, pulses are introduced probabilistically. Figure S3: Modeling of concentrations of CBG bound (pink), albumin bound (blue) and free cortisol (black) following a physiologically relevant 100 nmol/l pulse in blood already containing a 150 nmol/l total cortisol. The half-lives above reflect the differences in binding affinity, saturation and ability for 4 cortisol to be metabolized in each state. CBG has a stronger affinity for cortisol and therefore increases the half-life compared to free and albumin bound cortisol. Figure S4: The accumulation of cortisol in hair (pink) based on the pulsatile secretion of cortisol in the blood shown in blue and black. The accumulation in hair corresponds to a concentration of 25.8 pg cortisol/mg hair. Figure S5: Variability in total cortisol concentration (nmols/L) in blood across 500 simulations of 1 day, shown in 24-hour time. The horizontal bar indicates the mean concentration, the shorter vertical bar represents the 95% Confidence Interval and the longer vertical bar with points on the end represents the modeled distribution for cortisol at each time-point. Variability is based on changing baseline (as shown 5 in figure 2) and a probabilistic distribution of pulses introduced based experimental data obtained from the literature. 6 References 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Dorin, RI, Pai, HK, Ho, JT, Lewis, JG, Torpy, DJ, Urban, FK et al. Validation of a simple method of estimating plasma free cortisol: Role of cortisol binding to albumin. Clinical Biochemistry 2009; 42: 64-71. Young, EA, Carlson, NE,Brow, MB. Twenty-Four-Hour ACTH and Cortisol Pulsatility in Depressed Women. Neuropsychopharmacology 2001; 25: 267-287. Hruschka, DJ, Kohrt, BA,Worthman, CM. Estimating between- and within-individual variation in cortisol levels using multilevel models. Psychoneuroendocrinology 2005; 30: 698-714. Guo, W, Wang, Y,Morton, BB. A Signal Extraction Approach to Modeling Hormone Time Series with Pulses and a Changing Baseline. Journal of the American Statistical Association 1999; 94: 746-756. doi:10.2307/2669987. Finken, MJJ, Andrews, RC, Andrew, R,Walker, BR. Cortisol Metabolism in Healthy Young Adults: Sexual Dimorphism in Activities of A-Ring Reductases, but not 11 beta-Hydroxysteroid Dehydrogenases. The Journal of Clinical Endocrinology and Metabolism 1999; 48: 3316-3321. Gow, R, Thomson, S, Rieder, M, Van Uum, S,Koren, G. An assessment of cortisol analysis in hair and its clinical applications. Forensic Science International 2010; 196: 32-37. Linch, CA, Whitting, DA,Holland, MM. Human Hair Histogenesis for the Mitochondrial DNA Forensic Scientist. J Forensic Sci 2001; 46: 844-853. Magos, L,Clarkson, TW. The assessment of the contribution of hair to methyl mercury excretion. Toxicology Letters 2008; 182: 48-49. Stalder, T, Steudte, S, Miller, R, Skoluda, N, Dettenborn, L,Kirschbaum, C. Intraindividual stability of hair cortisol concentrations. Psychoneuroendocrinology 2011; In Press. Kraan, GPB, Drayer, NM,De Bruin, R. Kinetics of cortisol metabolism and excretion. a hypothetic model based on the cumulative urinary radioactivity in eight multiple pituitary deficient patients. J. Steroid Biochem. Molec. Biol. 1992; 42: 169-177. Snyder, WS, Cook, MJ, Nasset, GS, Karhausen, LR, Howells, GP,Tipton, IH. Report of the Task Group on Reference Man. Vol. 23 (Pergamon Press, 1974). Bacq, Y, Zarka, O, Brechot, J, Mariotte, N, Vol, S, Tichet, J et al. Liver function tests in normal pregnancy: A prospective study of 103 pregnant women and 103 matched controls. Hepatology 1996; 23: 1030-1034. Kirschbaum, C, Kudielka, BM, Gaab, J, Schommer, N,Hellhammer, DH. Impact of gender, menstrual cycle phase and oral contraceptives on the activity of the hypothalamus-pituitaryadrenal axis. Psychosomatic Medicine 1999; 61: 154-162. Perogamvros, I, Aarons, L, Miller, AG, Trainer, PJ,Ray, DW. Corticosteroid-binding globulin regulates cortisol pharmacokinetics. Clin Endocrinol 2011; 74: 30-36. 7