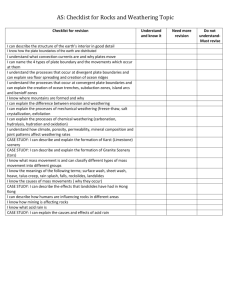
Activity 6 Do rocks last for ever (Weathering)
advertisement

Activity 5 - Weathering – rocks changing. adapted from Kids Geozone. Similar experiments can be found in Weathering and Erosion. The shaping of our landscape. Building Science Concepts 2 Science Understandings: Over time the interaction of air, water, temperature change, radiation and living things causes the outer layer of the earth’s surface to break down and crumble. This process is called weathering. There are two main types of weathering, mechanical and chemical. o Mechanical weathering (scouring, battering, cracking, levering) break up rock into smaller pieces. o Chemical weathering transforms rocks and minerals exposed to water and atmospheric gases into new chemical compounds and forms different rocks and minerals, some of which can be dissolved away (think of limestone caves). o In nature, mechanical and chemical weathering typically occur together. Weathering is a long, slow process, which is why we think rocks last forever. As rock is destroyed, valuable products are created. The major component of soil is weathered rock. The growth of plants and the production of food is dependent on weathering as it releases the minerals needed for these processes to take place. Resources: lemon juice, white vinegar, water water dropper bottles, pieces each of limestone, lime fertiliser, greywacke and quartz. Method: 1. Put a few drops of water on one of each of the samples. 2. Put a few drops of lemon juice and vinegar on one of each of the samples. 3. Look and listen carefully each time you add the water, lemon juice or the vinegar. 4. Record your observations on the chart below. Questions: What happens when you put water on each rock? What happens when you put lemon juice or vinegar on each rock? Did the lemon juice and vinegar act the same way on each rock? What about the water? Why did some of the rocks react differently? What does this experiment have to do with weathering? Water I saw Water I heard Lemon I saw Lemon I heard Vinegar I saw Vinegar I heard Greywacke Limestone Quartz Lime What should have happened: Lemon juice and vinegar are both weak acids and distilled water is neutral (not sour). The lemon juice contains citric acid and the vinegar contains acetic acid. These mild acids can dissolve rocks that contain calcium carbonate. The lemon juice and vinegar should have bubbled or fizzed on the limestone, and lime fertiliser, which all contain calcium carbonate. The lime fertiliser bubbles more, because this is ground up fine allowing the limejuice or vinegar to have more place to react. There should not have been a reaction on the greywacke or quartz, which do not contain calcium carbonate. Explain that rainwater commonly contains weak acids that dissolve rocks containing calcium carbonate and other minerals. You can google pictures of statues affected by acid rain.