taste_LECT07
advertisement

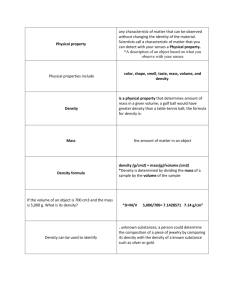
NEUROPHYSIOLOGY AND BIOCHEMISTRY OF TASTE "De gustibus non est disputandum. (There is no disputing in matters of taste.)" This remark of Julius Caesar reminds us of the subjectivity of taste perceptions, and the impossibility of deciding if one food tastes better than another. This probably reflects the modifiability of tastes, with age and state of health of the individual, drugs recently taken, other foods with which a given one is mixed, foods previously eaten, appetite, etc.. A given food, or tastant does not always produce the same sensation in everyone's mouth, or every time it is tasted. And food preferences may change with time, so that a taste once considered offensive (such as spinach or broccoli to many children) may become desirable to adults. Nature vs. Nurture in Taste Perception This brings us to the question, are tastes acquired as a result of various experiences during our lifetimes, or are they innate, or "inborn"? This is addressed in Figure 1. Three different neonates are shown, with facial expressions recorded after placing small amounts of various solutions in the infant’s mouth, presumably for the first time in his or her life. The first column is the unstimulated, or "resting" face. Column 2 is the response to distilled water (control). Column 3 is a solution of 25% sucrose; the response is described as an "expression of satisfaction." In Column 4 a sour solution of 2.5% citric acid is applied, resulting in pursing of the lips. Column 5 shows the response to 0.25% quinine sulfate (bitter solution). The expression is one of disgust; the upper lip is elevated, the angles of the mouth are depressed, and the tongue is protruded in a flat position, as if to expel the offending substance. All of these reactions are somewhat typical of adult behaviors to the same tastants, which suggests that the preference for sweet and avoidance of sour and bitter are inborn, and not learned. Having this ability to make judgements about tastes is advantageous since about 10% of plants are poisonous, and those plants also taste sour and/or bitter. Food which has decayed or fermented also tastes sour, and smells bad as well. On the other hand, almost all sweet-tasting plants are nutritious and nonpoisonous. Thus, the taste (or smell) of an unfamiliar food is usually a good indicator of its nutritive value or possible danger if consumed. It has been suggested that "gustofacial" expressions which a baby makes like those in Figure 1 are nonverbal communications to the mother to signal acceptance or dislike of some food. Probably the major sensation with consumption of food is one of smell. For example, all meats taste salty - chicken is different from beef or lamb only because of its odor (and its consistency). Fruits taste sweet and/or sour - you can try this at home: plug your nose and eat two differently flavored jelly beans; without smell, you won’t be able to tell the difference. This observation has led to the general rule, formulated by Haller in the 18th century, that FLAVOR = TASTE + ODOR It is familiar to most of us how the enjoyment of food strongly decreases when olfaction is impaired, as with the nasal congestion due to a cold. Anatomy of Taste Cells In mammals, the physiological detection and discrimination of various tastes occurs, in groups of cells located in the taste buds. These are situated on the sides and in clefts of structures known as papillae. The major types of papillae on the mammalian tongue are shown in Figure 2. Part A shows a large fungiform (or "mushroom-shaped") papilla surrounded by many filiform ("wireshaped") papillae. In the adult, taste buds are restricted to the sides of the fungiform papilla, and the filiform papillae do not have taste buds. Part B shows foliate ("leaf-shaped") papillae on the side of the tongue. The taste buds are located in the clefts between the papillae. In Part C a circumvallate (often called "vallate") papilla is shown. These are found in the posterior third of the tongue. The taste buds are located in the clefts of these papillae, also. Most of the tastebuds are in the back of the mouth: There are from 1 - 7 tastebuds in the fungiform papillae on the front of the tongue and as many as 400 each in the circumvallate papillae in the back. Bartoshuk and her collaborators at Yale University (Reedy et al., 1993; Bartoshuk et al., 1994) have shown that about onefourth of the population have an excess density of taste papillae on their tongues and perceive all tastes more strongly than the rest of us. These people are called supertasters. They also report stronger sensations with oral irritants such as chili powder. To see if you are a supertaster, you can perform the following test: Dab a few drops of blue food coloring on your tongue and swish it around. The pink circles that are exposed are the fungiform papillae. Make round hole in a piece of paper with a hole punch and place the paper on your tongue. (This hole is about 7 mm in diameter, and has an area of about 0.39 cm2.) People who cannot taste the bitter compounds phenylthiocarbamide (PTC) or 6n-propylthiouracil (PROP) will have from 0-15 papillae in the circle. Moderate tasters will have from 15-35 papillae, and supertasters more than 35 papillae. Women are supertasters more frequently than men, and have more fungiform papillae than men. The structure of a single tastebud is shown in Figure 3. The structure is about 50 m in diameter, and contains approximately 100 cells of epithelial origin, most of which extend from the base to the taste pore, or opening into the oral cavity. It is in this pore area that tastant molecules in the mouth presumably interact with chemosensitive cells. Taste buds in the front of the tongue are more sensitive to salt and sweet stimuli and those in the back are more sensitive to sour and bitter. However, taste buds in all areas of the mouth can taste all of the basic tastes, and the taste buds in all areas of the tongue are considered to be indistinguishable by light microscopy. The cell types in a tastebud are usually classified on microscopic grounds as shown in Table I. Table I. Types of cells in vertebrate tastebuds (After Kinnamon et al., 1985 and Roper, 1989). _______________________________________________________________ Type Shape Nucleus Electron Inclusions density _______________________________________________________________ I (Dark) Long, narrow Irregular High II(Light) Long, narrow Oval Low III Oval Low Long, narrow Apical granules Basal dense core vesicles Basal Flat, oblate Oval High Electron-lucent and dense vesicles _______________________________________________________________ Type I cells make up about 60% of a mouse taste bud, and Type II about 30%. Some cells with properties intermediate between Types I and II are also seen. The dense core vesicles in Type III cells have been suggested to have a monamine storage function.In a study of cell lineage Delay et al. (1986) found that radioactive thymidine was incorporated first into basal cells, then Type I followed by intermediate cells and finally Type II cells. This result strongly suggests that the stem cells (basal cells) transform during their lifetimes into Type I cells, then intermediate cells and finally Type II cells. The lifetime of a taste cell is not very long. Beidler and Smallman (1965) showed that the average lifespan in the rat was about 10 days. (This compares to about 4-8 days in the surrounding non-chemosensitive epithelium.) So there is a continuous turnover of taste receptors, with constant replacement of synaptic connections to the associated sensory axons. Interestingly, the presence of sensory innervation is an absolute requirement for the formation of tastebuds - section of a taste nerve results in disintegration and absorption of the tastebuds, and flattening of the papillae. If the nerve later regrows into the tongue, a trophic factor again causes the tastebud structures to form. Neural Pathways for Taste From ultrastructural studies, the different cell types in vertebrate tastebuds have been shown to be synaptically connected as shown in Figure 4. Receptor cells (R), both Type I and Type II, are connected to each other and to basal cells (B). Both receptor cells and basal cells make synaptic contact with sensory axons (A). Thus, there is a possibility of interaction between taste cells at the receptor level, similar to the interaction between cells in the retina. The activity of all the connected cells then excites a sensory axon, which conducts the signal to the nervous system. The cranial nerves which carry taste signals are shown in Figure 5. The anterior 2/3 of the tongue is innervated by the chorda tympani branch of VII, and the posterior 1/3 by the IXth nerve. Taste buds around the epiglottis are innervated by the Xth nerve. No afferent taste fibers are specific to any one of the socalled four basic tastes - salty, sweet, sour and bitter. Instead, some afferents respond more strongly to one or more stimuli than do other fibers. The pattern of activity of thousands of such axons is decoded in central structures to identify the tastant stimulus (see for instance Erickson, 1963). Some older textbooks show the sense of taste as being represented only on the tongue. There are also many tastebuds on the hard and soft palate (Henkin and Christiansen, 1967). The senses of touch, pain and temperature in the anterior 2/3 of the tongue are carried by the Vth nerve, which is also the route of perception of irritants such as chili powder. (This is sometimes known as the common chemical sense.) These sensations are represented in the posterior 1/3 of the tongue mainly by the IXth nerve and by the Xth nerve near the epiglottis. Primary afferent nerves in the taste pathway synapse in the nucleus of the tractus solitarius, which is also an important sensory nucleus for swallowing (Chapter 15). Axons from cells in this nucleus project to the ventral posterior median nucleus of the thalamus, and then to the gustatory neocortex, as shown in Figure 6. This is located in the portion of the postcentral gyrus where the head is represented, near the temporal gyrus. This has been determined from electrical stimulation of the cortex, which results in taste sensations, and from bullet wounds which interfere with perception of tastes. Chemical Structure and Taste It is generally agreed that almost everything we can taste falls under the categories known as the four basic tastes - salty, sweet, sour and bitter. (Some workers also describe the water taste, or taste of pure H2O, and the metallic taste, or taste of metals such as iron applied to the tongue, as distinct and recognizable sensations. There is apparently also a unique taste called umami, which is the taste of things meaty and savory.) The fact that different compounds only produce basic-taste sensations suggests that there are approximately four types of taste receptors on the chemosensitive cells in the mouth (Although recent genetic studies suggest a large multi-gene family of receptors for bitter taste - Adler, et al., 2000). Let us briefly consider the kinds of chemical stimuli for which each class of receptors is specialized. Salty Table II shows a list of things which taste salty. The pure salt taste is that of sodium chloride. Potassium is used as one component of salt substitutes, but by itself has a bitter taste at high concentrations. Table II. Salty-tasting compounds ________________________________________________________________ General form: electrolyte A+B- Sodium chloride NaCl Lithium chloride LiCl Sodium bromide NaBr Calcium chloride CaCl2 Ammonium chloride NH4Cl ________________________________________________________________ Sweet The sweet taste is more complicated, as shown by some of the compounds listed in Table III. There is evidence that these act on the same set of receptors, since the sweet taste of all of them is blocked by pre-treatment of the tongue with gymnemic acid, an extract of the plant Gymnema sylvestre (Bartoshuk et al., 1969). This compound tastes like strong tea, and rinsing the tongue blocks all sweet tastes for about one hour without affecting salt, sour or bitter. The gymnemic acid occupies the sweet receptors in the mouth, preventing other sweet-tasting compounds from stimulating them. Table III. Sweet-tasting compounds ________________________________________________________________ Sugars OHC-HCOH-HOCH-HCOH-HCOH-CH2OH Alcohols OH-CH2-CH2-OH Glycols HO-C=C-OH Saccharin Leucine CO / \ / \ | | N \ / \ / SO2 (CH3)2-CH-CH2-CH(NH2)-COOH Lead acetate Pb(CH3-COO -)3 Beryllium chloride BeCl2 Aspartame N-Aspartylphenylalanine _______________________________________________________________ The sweet taste may be due to the formation of a double hydrogen bond between the tastant molecule and receptor complex on the surface of the taste cell, as shown in Figure 7. This theory was put forth by Shallenberger (1971), and succeeded in explaining the sweet tastes of several different compounds, some ionized and some not. It is assumed that the tastant has a hydrogen-donating site within 3 Ångstroms of a hydrogen-accepting site, and that these have complementary donating and accepting sites on the receptor membrane, also spaced 3 Ångstroms apart. For instance, in saccharine the nitrogen atom can donate a hydrogen ion and the oxygen can accept. Metal compounds in solution are hydrated and in this configuration can donate and accept H-ions. The federal government has now recognized the carcinogenic properties of saccharin and requires that it be labeled accordingly. Some caution is also called for with aspartame, a widely-used sweetener, but for a different reason: Aspartame is composed of aspartic acid and phenylalanine, the second of which is an essential amino acid. However, phenylalanine also is an excitotoxin, and causes destruction of nerve cells at excessive concentrations (Olney, 1975). In phenylketonuric babies, the inability to metabolize phenylalanine leads to destruction of brain tissue and mental retardation. Wurtman (1983) has shown that the consumption of three cans of a soft drink sweetened with aspartame can double the blood concentration of phenylalanine. It is probably advisable to limit the amount consumed, especially by very young children. Sour The sour taste is the best understood, and is due to the H+ ion or proton. Not all acids are equally sour, but all acids taste sour, as shown in Table IV. When the sense of smell is excluded, equi-sour concentrations of different acids are indistinguishable. Table IV. Sour-tasting compounds ________________________________________________________________ General form: acid H+B- Hydrochloric acid HCl Sulfuric acid H2SO4 Nitric acid HNO3 Acetic acid CH3COOH ________________________________________________________________ Bitter Finally, the bitter taste is familiar from drinks containing quinine, and some bitter-tasting compounds are shown in Table V. Plant alkaloids usually have a bitter taste, but so do urea and potassium chloride. Some investigators have suggested that the bitter taste is also stimulated by a double hydrogen-bond mechanism, but that the hydrogen-donating site is closer to the hydrogen-accepting site than with sweet receptors, perhaps 1.8 Ångstroms. Table V. Bitter-tasting compounds ________________________________________________________________ Quinine C20H24N2O2.3H2O Caffeine C8H10N4O2 Nicotine C10H10N2 Urea H2N-CO-NH2 Potassium chloride 0.1 M KCl ________________________________________________________________ This theory can explain some interactions known to occur between the sweet and bitter tastes: (1) After adapting sweet receptors with strong sugar solution, distilled water has a bitter taste. (2) Saccharin has a bitter aftertaste to many people. (3) The dihydroxy alcohols (HO-C-C-..-C-OH) change from a pure sweet taste with two carbon atoms between the hydroxyl groups to a mixed sweet-bitter taste with three or four carbons, and a pure bitter taste with five carbons. In this case, the hydrogen-donating and accepting sites (OH) on the tastant molecule may be about 3 Å apart in the shorter alcohol and then fall closer together with the increased folding of the larger 5-carbon molecule. ELECTROPHYSIOLOGY OF TASTE CELLS Previously the term "receptor" has been applied to receptor cells in tastebuds (cf. Figure 4). Nowadays, it is usually restricted to mean chemosensitive groups on the surface of taste cells. These are located in the area of the taste pore of each tastebud, where the receptors and active ionic channels are also found (see, for instance, Kinnamon, 1992). Taste cells have been compared to postsynaptic nerve or muscle membranes (Roper, 1989), because they contain active ion channels and receptors for hormones, neurotransmitters and other dissolved substances, both organic and inorganic. For many years, taste cells were considered electrically inexcitable (Tateda and Beidler, 1964; Akaike et al., 1976; Sato, 1980). This finding was probably a result of the relatively largediameter microelectrodes which were originally used to record from the cells. When finer microelectrodes and whole-cell patch electrodes were used, the ability of taste cells to produce regenerative action potentials was confirmed (Roper, 1983; Kinnamon and Roper, 1987; Avenet and Lindemann, 1987a). More recently, the voltage-clamp method has been used to measure ionic currents flowing across taste cell membranes during stimulation with various compounds (Avenet and Lindemann, 1987b; Kinnamon and Roper, 1988a; Sugimoto and Teeter, 1990). From these studies, a clearer picture of the mechanisms of excitation by various tastants is emerging. One apparently uniform observation is that all of the basic-taste stimuli excite the attached sensory axons by Ca-dependent release of neurotransmitter from the taste cell into the synaptic cleft. How this is achieved differs from taste cell to taste cell. Salty The current evidence suggests that NaCl (or another salty compound, such as LiCl) stimulates taste cells by direct entry through passive (ungated) channels in the cell membrane. This is shown schematically in Figure 8, Part A. One line of work pointing to this idea is that application of amiloride, a passive Nachannel blocker, suppresses the response of the chorda tympani nerve to sodium applied to the tongue (Brand et al., 1985; DeSimone and Ferrell, 1985). Amiloride also reduces the taste intensity of Na and Li in humans (Schiffman et al., 1983). The sodium which enters the cell presumably depolarizes it until the threshold is reached for regenerative action potentials, which in turn triggers an influx of calcium through voltage-gated Cachannels (Avenet, 1992). Sweet With sweet compounds, there are some structural similarities which suggest that they interact with specific receptors. (Also, the receptors are specifically blocked by gymnemic acid.) Since most sweet stimuli are nonpolar compounds, no direct ionic mechanism of excitation such as that for salty stimuli may be postulated. Instead, the operation of a second messenger system is proposed, as indicated in the following scheme (Roper, 1989): Sweet stimulus activates GTP-binding protein stimulates adenylate cyclase increases intracellular cAMP (or cGMP) closes apical K+ channels depolarizes taste cell opens voltage-dependent Ca2+ channels allows influx of Ca2+ near synapses causes neurotransmitter release near synapses Treatment of some cells in the mouse tastebud with sucrose causes a depolarization and decrease in resting K-conductance (Tonosaki and Funakoshi, 1988). Injection of cAMP or cGMP into these cells produces the same effects. Avenet and Lindemann (1987b) also showed that external or internal application of cAMP to frog taste cells caused a reversible depolarization due to blockage of Kcurrents. Sour As shown in Figure 8, Part B, the effect of acids on taste cells is probably to depolarize by decreasing the currents through the voltage-gated potassium channels. It had been known for some time that acids produced depolarizations in taste cells (Akaike et al., 1976; Tonosaki and Funakoshi, 1984). Kinnamon and Roper (1988a,b) showed that the acid-induced depolarization in mudpuppy taste cells was due to a reduction in K-conductance, and the effect was blocked by application of the K-channel blocker TEA (tetraethylammonium. The effect of lowering pH on single potassium channels is shown in the next figure. This, like the salt response, is considered a direct ionic effect on the membrane channels, not requiring a specific macromolecular receptor. Molecular Biology of Taste Perception Some recent work by Margolskee and his collaborators has shown a taste-specific G-protein in extracts of tastebud tissues. The -subunit of the G-protein, called -gustducin, is 90% genetically similar to transducin seen in retinal cones, and thought to mediate light perception. The proposed function of gustducin in taste is shown in the slide. Gustducin is stimulated by taste stimulants binding to the receptor, and activates a phosphodiesterase that reduces the amount of intracellular cyclic GMP, causing the Ca-channels to open and admitting Ca, leading to transmitter release to the attached sensory nerves. Some of the evidence for this theory comes from knockout mice that lack the gene encoding -gustducin. These animals can taste salty and sour compounds but are unable to taste sweet or bitter things. This result also shows a connection between sweet and bitter. Bitter Some bitter stimuli are lipophilic and some are hydrophilic, so they may either enter the taste cell or not. Thus, a specific receptor may not be necessary. However, the existence of taste blindness for certain bitter stimuli such as PTU (phenylthiourea) may indicate some specificity of the receptor or the transduction process (Teeter and Cagan, 1989 Zuker and colleagues (Adler et al., 2000) at UC San Diego have identified a family of 40-80 G protein-coupled receptors (called T2R receptors) expressed in taste receptor cells. These are likely to be bitter taste receptors as (1) they are genetically linked to loci that influence bitter taste in mice and humans, and (2) mice deficient in the associated mT2R-5 gene are poorly able to perceive bitter tastants (Chandrashekar et al., 2000). The next figure shows calcium increases produced in cells expressing T2R receptors in the presence of the bitter compounds cycloheximide and denatonium. Interestingly, T2R receptors are only expressed in cells that contain gustducin, again suggesting a role for that G protein in both sweet and bitter tastes. In the case of the bitter taste, is appears there are receptors for many different kinds of bitter compounds, all of which taste bitter. _______________________________________________ While it is interesting to interpret the responses of single taste cells to the four basic-taste stimuli, these are often complex and the whole story is probably not as straightforward as outlined above. In the next chapter we shall consider some modifications of taste sensations produced by a variety of developmental, systemic and environmental variables.