General Molecular Bi..
advertisement

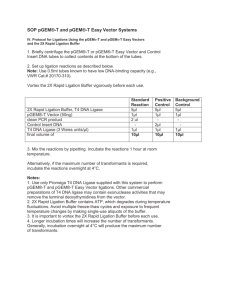
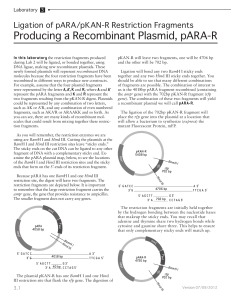
Last Updated: 02/17/16; 2:16 AM General Molecular Biology Protocols Transformed Cells: Store cells in 15% (or greater) Glycerol at –80°C. Store cells in 3.5% DMSO at –80°C. Pouring LB plates: 1. LB with agarose is made up in approx 1L and autoclaved with stir bar (approximately 30 minutes). 2. Flask is then placed in 50°C waterbath so that Ampicilian (concentration ???) can be added. 3. Pour LB/Agarose into plate so that the bottom is covered. Stack poured plates in groups of 10 to decrease condensation as they cool. 4. Allow to cool overnight at room temperature. 5. Plates are marked with a black stripe to indicate Amp resistance. Transformation: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. Thaw competent cells (50l volume) Place AMP+plate at 37°C Add 10 ul of plasmid to cell tube Incubate tube on ice for approx 30 min Heat shock cells (37° or 42°C) for 20 sec Place cells on ice for a few min Bring volume up to 1ml with LB media Shake at 37°C for 30 min to 1 hour Spin cells down for 30 sec Remove approx 900 ul (disgard) Plate remaining volume and grow overnight 37°C Pick colonies and culture for miniprep D:\533569420.doc Last Updated: 02/17/16; 2:16 AM Plasmid 10 Minute Miniprep Solutions: 1) 2) 3) TENS (make fresh each time): To 4.5 ml TE add: 250 ul 10% SDS 250 ul 2N NaOH Sodium Acetate 3.0M; pH 5.5 TE with 10ug/ml of RNAse A Procedure: 1. Spin 1.5 ml of overnight culture for 30 sec in microfuge 2. Aspirate off all but 100ul of the supernatant and resuspend the pellet by vortexting. 3. Add 300ul of TENS and mix by inversion. The solution should become viscous. 4. Add 150ul of sodium acetate and vortex. A fine white precipitate should form. 5. Centrifuge for 2.5 minutes at 10K. 6. TRANSFER the supernatant to a clean tube and add 2 volumes (1ml) of room temperature EtOH. 7. Vortex and pellet DNA by centrifugation for 2-5 minutes at 10K. 8. Wash pellet with 70% ethanol and allow the pellet to dry. 9. Resuspend the pellet in 30ul of TE with RNAseA. 10. Digest 5-10ul as usual. D:\533569420.doc Last Updated: 02/17/16; 2:16 AM Example Digestion Reaction – 20 l reaction volume Buffer (10X) Restriction Enzyme DNA dH2O 2 ul 1 ul 6 ul 11 ul if BSA is required by enzyme, reduce amount of water added. 0.7% Agarose Gel (Buttrick Lab) for 300 ml: 2.1 grams of SeaKem Agarose 300 ml of 1X TAE 21 ul of EthBr (add once agarose is cooled to < 60°C) Maxiprep Grow up 500 ml culture with AMP in a 2000 ml flask overnight. Prepare via Qiagen kit. Check purity and concentration of DNA by absorbance at 240, 260, 280. Purity is determined by A260/A280 and should be above 1.5. Concentration is determined by the following: A260 * 50 * dilution / 1000 = X ug/ul. Gel Purification of DNA fragments Run approximately 2-4 ug of DNA on 0.7% gel to isolate desired fragment. Ligation Reaction (eg. preferably use 10 ul reaction volume) Ligation Ligation + T4 DNA Ligase 1 ul T4 DNA Ligase Ligase buffer (5X) 2 ul Ligase buffer (5X) Vector 2 ul Vector dH2O 5 ul Insert Incubate 1 hour at room temp and then at 14°C overnight use approximately 3:1 of insert to vector for ligation. D:\533569420.doc 1 ul 2 ul 2 ul 5 ul