Volcano Demo Ideas for Chemistry/Geology
advertisement

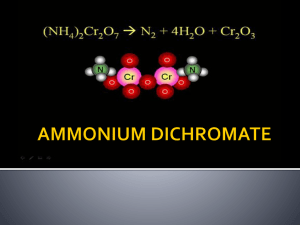
Volcano Demo Ideas for Chemistry/Geology Retrieved 3/28/05 from http://chemistry.miningco.com/cs/howtos/ht/buildavolcano.htm How To Build a Volcano Okay, it's the kitchen equivalent of a volcano, not a real one. It's cool all the same! It's also more or less non-toxic, which adds to its appeal. Difficulty: Average Time Required: 30 minutes Here's How: 1. First make the 'cone' of the volcano. Mix 6 cups flour, 2 cups salt, 4 tablespoons cooking oil, and 2 cups of water. The resulting mixture should be smooth and firm (more water may be added if needed). 2. Stand the soda bottle in the baking pan and mold the dough around it into a volcano shape. Don't cover the hole or drop dough into it! 3. Fill the bottle most of the way full with warm water and a bit of red food color (can be done before sculpting if you don't take so long that the water gets cold). 4. Add 6 drops of detergent to the bottle contents. 5. Add 2 tablespoons baking soda to the liquid. 6. Slowly pour vinegar into the bottle. Watch out - eruption time! 7. Chemistry is Cool :-) Tips: 1. 2. 3. 4. The cool red lava is the result of a chemical reaction between the baking soda and vinegar. In this reaction, carbon dioxide gas is produced, which is also present in real volcanos. As the carbon dioxide gas is produced, pressure builds up inside the plastic bottle, until the gas bubbles (thanks to the detergent) out of the 'volcano'. Adding a bit of yellow food coloring too will result in a lovely lava red-orange! What You Need: 6 cups flour 2 cups salt 4 tablespoons cooking oil warm water plastic soda bottle dishwashing detergent food coloring vinegar baking dish or other pan 2 T baking soda Retrieved 3/28/05 from http://chemistry.about.com/cs/demonstrations/a/aa033003a.htm How to Make the Classic Chemical Volcano Ammonium Dichromate Reaction Introduction The eruption of an ammonium dichromate [(NH4)2Cr2O7] volcano is a classic chemistry demonstration. The ammonium dichromate glows and emits sparks as it decomposes and produces copious amounts of green chromium (III) oxide ash. This demonstration is simple to prepare and perform. The decomposition of ammonium dichromate commences at 180°C, becoming self-sustaining at ~225°C. The oxidant (Cr6+) and the reductant (N3-) are present in the same molecule. (NH4)2Cr2O7 --> Cr2O3 + 4 H2O + N2 The procedure works well in both a lighted or darkened room. Materials ~20 grams of ammonium dichromate sand tray or ceramic tile, for use in ventilation hood OR 5-liter round bottom flask and porcelain filtering funnel gas burner (e.g., Bunsen) OR butane lighter or match, for use with flammable liquid (e.g., ethanol, acetone) Procedure If you are using a hood: 1. 2. Make a pile (volcanic cone) or ammonium dichromate on a tile or tray of sand. Use a gas burner to heat the tip of the pile until the reaction begins or dampen the tip of the cone with a flammable liquid and light it with a lighter or match. If you are not using a ventilation hood: 1. 2. 3. Pour the ammonium dichromate into a large flask. Cap the flask with a filtration funnel, which will prevent the majority of the chromium (III) oxide from escaping. Apply heat to the bottom of the flask until the reaction begins. Notes Chromium III and chromium VI, as well at its compounds, including ammonium dichromate, are known carcinogens. Chromium will irritate the mucous membranes. Therefore, take care to perform this demonstration in a well-ventilated area (preferably a ventilation hood) and avoid skin contact or inhalation of the materials. Wear gloves and safety goggles when handling ammonium dichromate.