Energy saving lightbulb OW task
advertisement

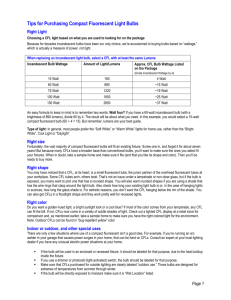
Energy Saving Light Bulbs This Bulb. (2007, August 17) National Geographic. Retrieved January 13 2011, from http://www.youtube.com/watch?v=FvOBHMb6Cqc&feature=related Energy Saving Lights A compact fluorescent lamp (CFL), or energy saving light, is a type of fluorescent lamp. They are designed to replace a (normal) incandescent bulb and can fit into most existing light fixtures. Compared to a normal bulb, CFLs use less power and have a longer life. A CFL has a higher purchase price than an incandescent lamp, but can save over US$40 in electricity costs over the lamp's lifetime. Like all fluorescent lamps, CFLs contain mercury, which makes them difficult to dispose of. CFLs radiate a different light spectrum from that of normal bulbs. Improved phosphor formulations have improved the colour of the light emitted by CFLs. Some sources rate the best 'soft white' CFLs as subjectively similar in color to standard incandescent lamps. adapted from: Compact fluorescent lamp. (2012, November 19). In Wikipedia, The Free Encyclopedia. Retrieved November 21, 2012,from http://en.wikipedia.org/w/index.php?title=Compact_fluorescent_lamp&oldid=523872987 CFL bulbs The incandescent light bulb hasn't changed much since Thomas Edison introduced it in 1879. Even today, it still generates light by heating a tungsten filament until it reaches 2,300 degrees Celsius and glows white-hot. Unfortunately, much electricity - electricity from coal-fired powered plants responsible for spewing greenhouse gases into the atmosphere - is required to make an incandescent bulb burn brightly. Only 5% of it goes toward making light. The rest is wasted as heat. Luckily, there's a new type of light bulb known as the compact fluorescent light bulb, or CFL, and its illumination comes by way of a very different mechanism. Instead of a glowing filament, CFLs contain argon and mercury vapor housed within a spiral-shaped tube. Believe it or not, CFLs are closely related to the lightsaber-shaped fluorescent bulbs that still flicker in garages and workshops all over the world. They also have a device (ballast) to produce an electric current to pass through the mixture, exciting the gas molecules. In older CFLs, it took several seconds for the ballast to produce enough electricity to increase the excitation. Newer CFLs have more efficient ballasts and require a shorter warm-up. Either way, when the gas gets excited, it produces ultraviolet light. The ultraviolet light, in turn, stimulates a fluorescent coating painted on the inside of the tube. As this coating absorbs energy, it emits visible light. Compact fluorescent light bulbs deliver their best results when left on for 15 minutes or longer. Switching CFLs on and off will shorten their life and may decrease their efficiency, mainly because the excitation of the gases and of the fluorescent coating take some exposure to an electric current to reach an optimal level. CFLs are also inefficient in enclosed, recessed fixtures (too hot) and in the fixtures of garage-door openers (too much vibration). Finally, CFL bulbs can, in rare cases, interfere with electronic equipment. This interference is caused by infrared (IR) light, which CFLs produce and which IR readers can interpret as a signal. However, CFLs use significantly less energy - 75 percent less energy than incandescent light bulbs. That means CFLs require less wattage to produce an equivalent amount of light. For example, you could use a 20-watt CFL and enjoy the same amount of light as a 75-watt incandescent. If every home in America made one such swap, enough energy would be saved in one year to light more than 3 million homes. Of course, if you're using less energy, your energy costs are going to go down. Replacing a standard 60-watt bulb with a 13-watt CFL can save a single household $30 in energy costs over the life of the bulb. Even with the higher price tag of CFLs -- you'll pay $2 to $4 for a CFL versus 30 to 40 cents for a typical incandescent bulb- they still save you money. That's because CFLs last a long time. In some tests, they burned brightly for 10,000 hours, whereas standard bulbs burned for just 800 to 1,500 hours. A Quicksilver Lining Mercury is a persistent and highly toxic chemical. Most humans are exposed to the poison by eating fish contaminated with methyl mercury. However, it's also possible to inhale elemental mercury vapor, like the kind used in compact fluorescent light bulbs. When stimulated by electric current, mercury vapor inside a CFL produces ultraviolet light, which is re-radiated as visible light when it strikes the fluorescent compound, known as phosphor, painted on the inside of the bulb. No other element has proved as efficient in this process, so even though the amounts of mercury used in bulbs has decreased over time, a small amount of mercury is still required for CFLs to function properly. One CFL bulb typically requires approximately 5 milligrams of mercury. A broken CFL bulb, however, can expose a person to mercury vapor. A tiny amount of solid mercury powder can also be released. For these reasons, extra caution should be taken when cleaning up a broken CFL. So what do you do with a burned-out CFL bulb if it contains mercury? Compact fluorescent bulbs are considered hazardous household items, a category that also includes paint, batteries and thermostats. You must dispose of such items properly, which means you can't simply throw them away in household garbage. As recycling becomes easier, the CFL revolution will only gain momentum. Many think that the spiral-shaped bulbs will be a stage toward the even more efficient light-emitting diode (LED) bulbs. A 1.3-watt LED bulb uses less electricity than a 9-watt CFL and can last for up to 11 year, and it doesn't contain an atom of mercury. But even LED bulbs have their tradeoffs. They're not very bright, and they're very expensive. Until manufacturers overcome those two issues, CFL bulbs will likely have the spotlight all to themselves. Harris, William. How CFL Bulbs Work. (2009, July 14). In HowStuffWorks.com. Retrieved November 21, 2012,from http://science.howstuffworks.com/environmental/green-tech/sustainable/cfl-bulb.htm Name ……………………….. CFL bulb One World Activity Watch the short video and read the articles to help answer the questions about this application of science. 1. What problem is the CFL bulb designed to solve? ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. 2. How does the CFL bulb work? ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. 3. What is one benefit of the CFL bulb? ……………………………………………………………………………………………………………….. Details……………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. 4. What is another benefit of the CFL bulb? ……………………………………………………………………………………………………………….. Details……………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. 5. What is one problem that the CFL bulb doesn't solve? ……………………………………………………………………………………………………………….. Details……………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. 6. What is another problem that the CFL bulb doesn't solve? ……………………………………………………………………………………………………………….. Details……………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. 7. How effective do you think the CFL bulb is as a solution to the problem? ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. Resources and references Comment on the reliability of each of the resources 8. This Bulb ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. 9. Wikipedia “Compact fluorescent lamp” ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. 10. How CFL bulbs work ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………….. References Compact fluorescent lamp. (2012, November 19). In Wikipedia, The Free Encyclopedia. Retrieved November 21, 2012,from http://en.wikipedia.org/w/index.php?title=Compact_fluorescent_lamp&oldid=523872987 Harris, William. How CFL Bulbs Work. (2009, July 14). In HowStuffWorks.com. Retrieved November 21, 2012,from http://science.howstuffworks.com/environmental/green-tech/sustainable/cfl-bulb.htm This Bulb. (2007, August 17) National Geographic. Retrieved January 13 2011, from http://www.youtube.com/watch?v=FvOBHMb6Cqc&feature=related Science One World Task Name ……………………… Compact Fluorescent lights A One world Maximum 6 Level 1-2 Level 3-4 Level 5-6 How is science used to solve problems? state how science is applied and how it may be used to address a specific problem or issue in a local or global context describe how science is applied and how it may be used to address a specific problem or issue in a local or global context explain how science is applied and how it may be used to address a specific problem or issue in a local or global context How good are scientific solutions at solving problems? state the effectiveness of science and its application in solving the problem or issue describe the effectiveness of science and its application in solving the problem or issue discuss the effectiveness of science and its application in solving the problem or issue describe the implications of the use and application of science interacting with at least one of the following factors: moral, ethical, social, economic, political, cultural and environmental discuss and evaluate the implications of the use and application of science interacting with at least two of the following factors: moral, ethical, social, economic, political, cultural and environmental Level: How is society affected by science? Comment