Answers
advertisement

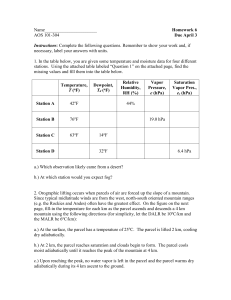
EAS 6140 Thermodynamics Worksheet 7– Combined 1st and 2nd Law 14. Write the first and second law combined in enthalpy form (intensive) dh Td vdp 15. The Gibbs energy is defined as g = u - T pv Write an expression for the Gibbs energy in differential form dg du ( dT Td ) pdv vdp 16. Using the expressions in #14 and #15, write an expression for the Gibbs energy in differential form that has natural independent variables T, p. dg dT vdp du= T d – p dv dh= T d + v dp dg= – dT + v dp 12. Write the natural independent variables for: internal energy: entropy and volume enthalpy: entropy and pressure Gibbs function: temperature and pressure 13. The conditions for thermodynamic equilibrium are: a) at constant T and p: dg = 0 b) at constant and p: dh = 0 c) at constant and V: du = 0 17. Match the thermodynamic equilibrium conditions: ___c_____ constant , v a. dg = 0 ___b_____ constant , p b. dh = 0 ___a_____ constant T, p c. du = 0 Potential temperature and the second law We previously derived equation (2.22): T2 T1 = p2 p1 Rc p 12. Which of the following equations were used in this derivation (circle all that apply) a) first law of thermodynamics b) second law of thermodynamics c) ideal gas law d) hydrostatic equation 13. Which of the following assumptions were made in this derivation (circle all that apply) a) adiabatic b) isothermal c) isobaric d) reversible The potential temperature, , for the atmosphere is defined as p = T p0 Rc p where po is 1000 hPa (mb). The potential temperature may be looked upon as the temperature of a sample of gas would have if it were compressed (or expanded adiabatically from a given state p and T to a reference pressure of 1000 hPa (mb). Refer to p 66. 14. Take the log of the potential energy equation, then write in differential form (i.e. in the left hand side you will have d(ln) ln ln T R R ln p0 ln p cp cp d (ln ) d (ln T ) R d (ln p) cp 15. Write the first and second law combined in enthalpy form for an ideal gas (2.3.2) dh Td vdp 16. From the expressions in #14 and #15, write an expression that relates entropy to potential temperature. dh Td vdp v RT p RT dp c p dT p d c p d (ln T ) Rd (ln p) dh Td d (ln ) d (ln T ) R d (ln p) cp c p d (ln ) c p d (ln T ) Rd (ln p) d c p d (ln ) 17. If potential temperature remains constant, the entropy (increases, decreases, remains the same). Remains the same 18. The potential temperature remains constant for a sample of ideal gas under dry adiabatic ascent/descent (yes, no, sometimes) Yes 19. The potential temperature of a parcel of air with T=288 K at p=900 hPa (mb) is (greater than, less than, equal to) 288 K. Greater than EAS 6140 Thermodynamics of Atmospheres and Oceans Thermodynamic Charts Important Note – Because in viewing these charts you might see a slightly different value, this could lead to differences in calculation results. So use the numbers here in a relative comparison to your findings. Using the Stuve (or Skew-T) diagram, read off the temperature and determine the potential temperature at each of the following levels Level 1000 mb TFFR sounding T 27 300 OAK sounding T 20 293 900 mb 20 302 22 302.12 700 mb 10 313.4 9 312.28 500 mb -7 324.32 -9 321.88 Estimate dT/dz and d/dz for the following layers Level TFFR sounding T 1000-900 mb -10.2ºC/km 2.9 ºC/km OAK sounding T +3.0075ºC/km 13.7 ºC/km 700-500 mb -6.69ºC/km -6.28ºC/km 4.04 ºC/km 3.56 ºC/km Determine the change in T and of an air parcel initially at 900 mb if subjected to TFFR sounding OAK sounding T T a. adiabatic lifting of 100 mb -10 0 -10 0 b. adiabatic lowering of 100 mb +10 0 +10 0 c. radiative heating of 10oC +10 +10 +10 +10 d. radiative cooling of 10oC -10 -10 -10 -10 Sketch the following paths on the TFFR diagram a) A parcel of air is lifted dry adiabatically from an initial height of 900 mb to a height of 700 mb. The parcel is then lowered back down to the initial pressure. Label this path A. Is this path reversible? yes b) A parcel of air is lifted dry adiabatically from an initial height of 900 mb to a height of 700 mb. The parcel is then heated radiatively by 10oC at constant pressure. The parcel is then lowered back down to the initial pressure.Label this path B. Is this path reversible?no