Polymers 3/PH/AM
P O L Y M E R S 3 / P H / A M
L e c t t u r e 5 .
.
B l l e n d s a n d B l l o c k s
Neighbours, everybody needs good Neighbours;
With a little understanding, you can find the perfect blend:
Neighbours, should be there for one another;
That's when good Neighbours become good friends.
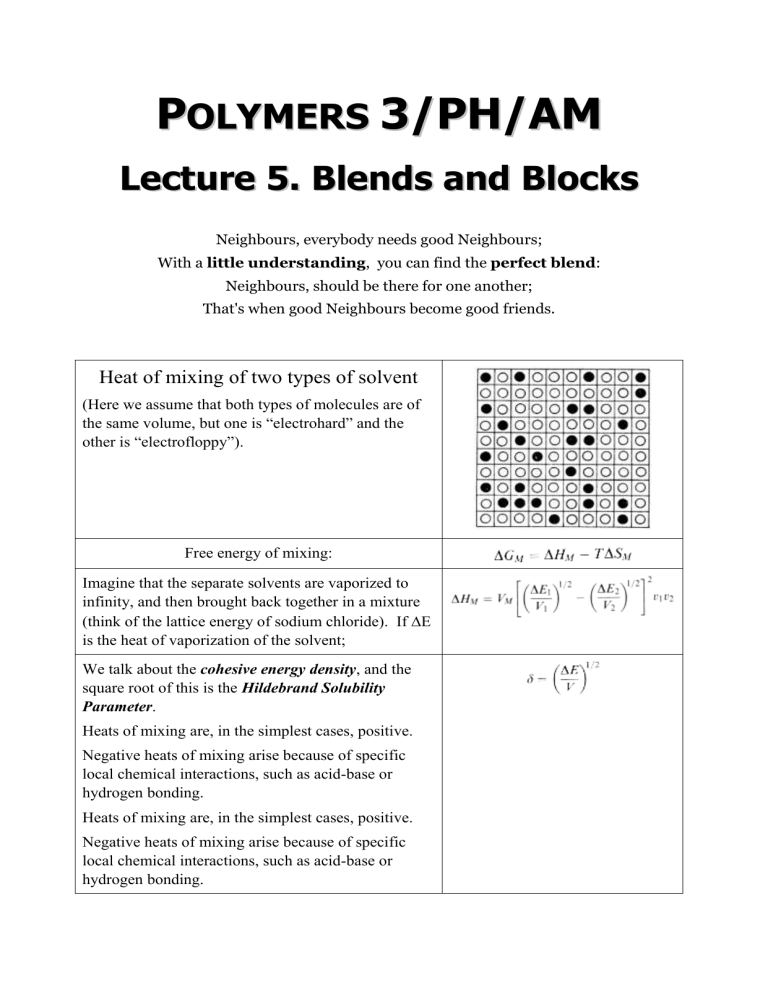
Heat of mixing of two types of solvent
(Here we assume that both types of molecules are of the same volume, but one is “electrohard” and the other is “electrofloppy”).
Free energy of mixing:
Imagine that the separate solvents are vaporized to infinity, and then brought back together in a mixture
(think of the lattice energy of sodium chloride). If
E is the heat of vaporization of the solvent;
We talk about the cohesive energy density , and the square root of this is the Hildebrand Solubility
Parameter .
Heats of mixing are, in the simplest cases, positive.
Negative heats of mixing arise because of specific local chemical interactions, such as acid-base or hydrogen bonding.
Heats of mixing are, in the simplest cases, positive.
Negative heats of mixing arise because of specific local chemical interactions, such as acid-base or hydrogen bonding.
For solvent-solvent on the left: where x
refers to mole fractions
For solvent-polymer in the middle, and for polymer-polymer on the right where
refers to volume fractions; note that x
or
<=1
, but N is big for solvents and small for polymers.
Free energy of mixing of two solvents:
Notice the “magic” value of 2
The same holds for polymers, but
‘concentration’ means volume fractions. If we are looking at two different polymers, both with N segments in the molecule, then read
N
for “chi”.
The Flory-Huggins theory for Blends
In order to keep it simple, we will examine the case of two very similar polymers with the same number of segments.
Then we get an entropy of mixing
and we get a free energy diagram like the above, except that for polymers, one needs to read N
for “Chi”.
Here again we have the “magic” value of 2
Lever Rule for Determining Proportions of Phases
Imagine we have a system with 2 phases present (labelled 1 and 2), and two types of polymers A and B.
In phase 1 concentration of A = c
1
In phase 2 concentration of A = c
2 and that there is x of phase 1 present and (1-x) of phase 2 present.
Total of N atoms, overall concentration of A = c therfore no of A atoms is Nc = Nxc
1
+ N(1-x)c
2
Thus, when one wants to find the average of some quantity such as F, it is usually sufficient
(neglecting surface effects) to take a weighted average.
Consider the free energy F as F(c)
F(c) = F2 + (F1 – F2) QR/PR
By similar triangles, F(c ) = SQ in the diagram.
Therefore one can find free energy of any intermediate composition by drawing straight line between the free energies of the two constituent phases.
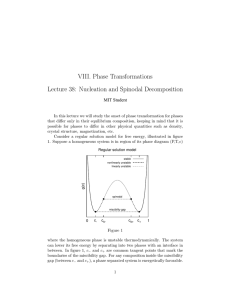
Necessary condition for homogeneous mixing to occur is for
However when N
>= 2, this inequality does not hold, and the behaviour is very different.
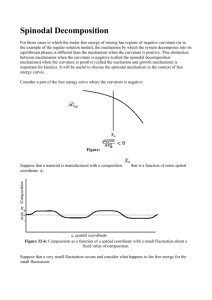
Phase separation occurs when free energy curve has regions of negative curvature.
Mixing when there is only one minimum in the free-energy curve
Mixing when there is more than one minimum in the free-energy curve
As pure components (A and B) F(c ) = F
This can be lowered by going to compositions A1,B1 to give free energy F1 etc.
And as A and B continue to dissolve more and more of each other, free energy continues to drop.
So there is a minimum energy for homogeneous single phase, energy Fn, i.e no phase separation occurs in this case.
Composition overall determines the state of the mixture.
This can split into A1 and B1; energy drops to F1. Minimum in energy occurs at F3 when the representative points on the free energy curve are joined by the lowest straight line: common tangent construction.
In this case, have phase separation into compositions c
A
and c
B
, with phases
and
.
For compositions c < c
A
have
phase c
A
< c < c
B
have
+
c
B
<c have just
.
Proportions of
and
given by Lever rule.
For c<c
A
A dissolves B
For c
B
<c B dissolves A c
A
and c
B
define solubility limits.
In the two phase region, two kinds of decomposition can occur.
The binodal or coexistence curve describes the limits of solid solubility.
Have seen condition for homogeneous mixing, namely d
2 f/dc
2
>= 0. The locus d
2 f/dc
2
= 0 is called the spinodal curve.
We need to distinguish how phase separation occurs inside this curve from
1. Nucleation and Growth occurs between binodal and spinodal. corresponds to a metastable region of the phase diagram
In the nucleation and growth regime at a nucleus of a critical size has to form before it is energetically favourable for it to grow.
2. Spinodal Decomposition
Spinodal decomposition occurs when any composition fluctuation is unstable. This is in the unstable region of the phase diagram.
Types of decomposition
Nucleation and Growth Spinodal Decomposition
Differences in Diffusion Mechanism
Coarsening Mechanism
This shows coarsening with time after spinodal decomposition
Example of Phase diagram for decomposition Upper Critical Solution
Temperature (UCST)
Block Copolymers https://webspace.utexas.edu/ylloo/www/images/pdf/Presentation3.pdf
Possible morphologies as B increases relative to A .
Note that here the critical value of
N
is about 10
(Matsen & Schick, 1994)