SUPPLEMENTAL MATERIAL Supplemental Methods Animal model
advertisement

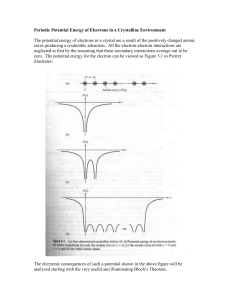
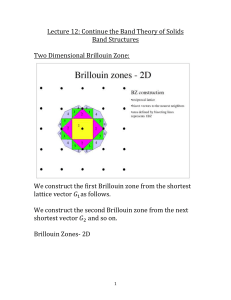
SUPPLEMENTAL MATERIAL Supplemental Methods Animal model of TCFA and histology One female ApoE-/- mouse was housed in the animal unit at Imperial College London. Animal care and all procedures complied with the Animals Act 1986 and were approved by the Home Office, UK. The mouse was initiated on a high-fat Western diet (Lillico Biotech, UK) at the age of 11 weeks and, 2 weeks later, a blood flow-modifying cuff (Promolding, Rijswijk, The Netherlands) was surgically placed around the left carotid artery. The cuff tapers from 500 m at the base to 250 m at the throat and creates a gradual artery stenosis with a maximum of ~75% by area, leading to three distinct regions of perturbed flow characterized by low wall shear stress (WSS) upstream, high WSS within, and low, oscillatory WSS (characterized by vortices) downstream of the cuff. Within 9 weeks of instrumentation, the cuff caused the development of TCFA in the segment upstream of the cuff, fibrous cap atheroma in the segment downstream of the cuff, and very little plaque within the cuff, as previously reported [1]. In this study, we focused on the TCFA in the upstream region. At 9 weeks, the mouse was sacrificed and the carotid arteries were harvested. Arteries were embedded in OCT and serially sectioned frozen at 8 µm intervals over the entire TCFA region upstream of the cuff. Sections were collected using an alternating methodology to allow assessment of the distribution of lipid (oil-red-O), collagen (picrosirius red), and stiffness (Brillouin shift) in neighbouring sections (i.e., oil-red-O and collagen staining were performed on sections that were 22.40±14.31 µm and 30.4±14.31 µm apart, respectively, from sections used for Brillouin measurements). Oil-red-O staining was done in 0.5% Oilred-O solution in Propylene glycol (Sigma Aldrich, UK) and a nuclear counterstain was done using Mayer’s Hemotoxylin (Sigma Aldrich, UK). After staining, all histology sections were imaged (MicroBeam 4.5 Pro laser capture microscope; Carl Zeiss, UK) at 10x magnification. Picrosirius red staining was done by immersing sections in Celestin Blue (Solmedia, UK) for 15 seconds, Harris Haematoxylin (Solmedia, UK) for 15 seconds, and picrosirius red (Sigma Aldrich, UK) for 1 hour with a tap water rinse between each step. Samples were then washed in acetic acid water, rinsed in tap water, and dehydrated through an ethanol series (70, 90, 100, and 100%). Sections were imaged using a light microscope with a polarizing filter at 10x magnification. Brillouin microscopy Spontaneous Brillouin scattering, first reported in 1922 [2], is a weak inelastic scattering process arising from the interaction of light with thermal acoustic waves (acoustic phonons) propagating at hypersound velocity in a medium. The spectrum of the scattered light contains the illumination frequency (Rayleigh peak) and two other frequencies, one blue- and one red-shifted with respect to the Rayleigh peak. The two peaks on either side of the Rayleigh peak and equal spectral distance (or shift) away from that, are referred to as the Brillouin peaks. The frequency shift of the Brillouin scattered light is given by , where (1) is the frequency of the illumination, c is the speed of light, n is the (local) refractive index, θ is the scattering angle (measured between the incident wave vector and the wave vector of the scattered light) and V is the hypersound velocity. Note that this equation either applies in general to isotropic media or specifically to a particular direction in anisotropic media. In isotropic media, the real part of the longitudinal modulus, also referred to as the Brillouin or longitudinal storage modulus M’, is given as , (2) where ρ is the material density. In anisotropic media there is a separate M’ in each direction corresponding to direction-dependent hypersound velocities. If an anisotropic sample with stiffness variations smaller than the optical resolution is measured in a Brillouin microscope, the resulting Brillouin shift will be due to the mean hypersound velocity within the probe volume. The width of the Brillouin peak on the other hand is related to the imaginary part of the longitudinal modulus, the so-called loss modulus. The simultaneous knowledge of storage and loss moduli together provides complete stiffness information of the sample at each pixel at the frequency of hypersound velocity. A recent study [3] found a linear relationship between the Brillouin modulus and Young’s modulus for a limited range of moduli. More precisely, Scarcelli et al. [3] used small volumes of fresh porcine and bovine eye lens tissue and carried out rheometer measurements to obtain Young’s moduli of elasticity for these samples. Subsequently, they carried out low resolution Brillouin microscope imaging of both samples to obtain the Brillouin frequency shift. The average of these values was then used to calculate M’ using Eqs. (1) and (2), which assume the knowledge of the refractive index, n, material density, ρ, and the scattering angle, θ. Whereas the last of the three parameters can be readily estimated in the case of low resolution measurements from the optical configuration of the microscope, the refractive index and material density are more difficult to measure. Scarcelli et al. elected to estimate these parameters; an approach that can be clearly justified when larger volumes of the sample are considered. Averaging over large volumes becomes particularly important in the case of anisotropic eye lenses because their direction dependent material properties would potentially yield very misleading data should the measurements involve small volumes. The authors also point out that, in general, moduli of elasticity depend on the frequency of the mechanical stimulus and so a significant difference is expected between moduli obtained by static rheometry, dynamic rheometry and Brillouin spectroscopy. Returning now to our present work, high resolution Brillouin microscopy provides information on the frequency shift in the following way. Due to the high numerical aperture (NA) illumination and detection, the scattering angle encompasses θ = 0 – 57° angle range, which causes the Brillouin peak to broaden and shift position [6]. This is not a problem in terms of the measurement accuracy because the effect is well understood; therefore, when significant, in some cases it can be calibrated out. Furthermore, the volume of the focused light (probe volume) that illuminates the sample is 0.435 µm3 so the measured frequency shift at each pixel is due to the average hypersound velocity within the probe volume. This again does not invalidate the data as long as it is clearly understood that, according to Eq. (1), pixel to pixel variations in the measured Brillouin frequency shift, may arise due to changes in either or both the average hypersound velocity and the refractive index. Therefore, in order to be able to calculate the hypersound velocity from the measured Brillouin shift one needs to determine the average reflective index within the probe volume. To appreciate how much error the assumption of uniform refractive index introduces, consider that the refractive index of the artery wall varies between 1.39 (intima), 1.38 (media), and 1.36 (adventitia) [7] whereas the refractive index of lipid within the plaque is 1.42 [8]. Therefore, since Eq. (1) reveals a linear dependence of the Brillouin shift on the refractive index; the maximum error one introduces within the artery wall is ~1.5% and between the artery wall and the lipid is ~4%. This error is at the detection limit of our system. A more significant problem arises when one is required to calculate the Brillouin modulus from the frequency shift. This calculation requires the knowledge of the material density within the probe volume, which is a quantity we have even less knowledge of than the refractive index. The other disadvantage of using elasticity moduli values to display the results of Brillouin microscope measurements is that the dimension of Brillouin modulus values (Pa) would encourage direct comparisons with other methods (e.g. AFM, optical elastography, microrheology, etc.). We have pointed out before, in agreement with Scarcelli et al. [3], that direct comparisons cannot be related to the elastic moduli obtained from different methods in a simple manner, particularly for anisotropic materials. Therefore, for purposes of this study, it is more reasonable to infer the stiffness obtained from the Brillouin microscopy data directly from the Brillouin frequency shift, instead of the hypersound velocity or Brillouin modulus (which we showed in our original submission). Future work, including the combined use of refractive index measurements and micromechanical simulations, will permit the calculation of an elastic modulus to allow computation of plaque stress distributions, which is the primary method of determining plaque vulnerability. To obtain the Brillouin frequency shift, a custom confocal Brillouin microscope was designed and built to acquire high spatial resolution images. Supplemental Figure 1 shows the diagram of the optical system. The light emitted by a Cobolt Jive DPSS single longitudinal mode laser (λ=561 nm) was focused on the sample by an oil-immersion microscope objective lens (100x, NA=1.3). The theoretical optical resolution values associated with our wavelength and numerical aperture are 300 nm (transversal) and 1.54 µm (axial). The light scattered by the sample was collected by the same lens and it was used in conjunction with another, lower NA lens to couple the light from the probe volume into a single mode optical fibre. The light exiting the fibre was collimated and subsequently incident upon the spectrometer. Spectral analysis was performed by means of a pair of crossed Virtually Imaged Phased Array (VIPA), resulting in an overall extinction of 60dB. The VIPA is a customised Fabry-Perot etalon that incorporates an anti-reflection (AR) coated entrance window to minimise optical losses [4,5]. The spectra were captured by an Andor Neo sCMOS camera set to 0.2s data acquisition time. Lorentzian function fitting was performed on the row spectra to aid the accuracy of Brillouin peak localisation. The Brillouin spectrum of water was used as a reference ( GHz) to permit calibration of the spectrometer. The sample was raster scanned by a Prior H117 motorised XY stage having 40nm step size. Correlation analysis The Brillouin microscope created a point-to-point Brillouin frequency shift (a measure of stiffness) map for each imaged vessel section. Each shift map was imported into a custom Matlab (version R2012a, Mathworks Inc., USA) program to segment the lumen, internal elastic lamina (IEL), and external elastic lamina (EEL). The average frequency shift could then be computed for each component of the vessel section, including the intima (or plaque region) and media (also referred to as wall, which is the space between the IEL and EEL; for control sections the intima could not be delineated, so the wall was the space between the lumen and EEL). In addition, the region within each Brillouin image between the lumen and EEL was broken into a grid of 30 equidistant points circumferentially and 20 equidistant points radially and the mean Brillouin shift was assigned to each grid point based on proximity (i.e., each grid point “represented” a local region or area within each vessel section and the mean Brillouin shift within that local region was assigned to the grid point). Histology sections were analysed similarly, via the following process (see Supplemental Figure 2 for a summary). First, each digitized histology section was also segmented to delineate the lumen, IEL, and EEL contours and the lipid stain was identified via a threshold (based on inspection of both the instrumented and control sections, which was then kept constant over all sections) using commercial software (Clemex Technologies, Canada). The contour and stain data were discretized (i.e., broken into small points) so that the centroid and area (area was only outputted for stain data) associated with each point could be exported to an XLS file. These data were then imported into Matlab and, identical to the discretization for the Brillouin shift map described above, the region between the lumen and EEL was broken into a grid of 30 equidistant points circumferentially and 20 equidistant points radially. Next, the stain data (x-, y- coordinates of each centroid and area in µm2) was assigned to each grid point based on proximity and stain areas were summed at each grid point. Since each grid point “represented” a local region or area of the vessel cross-section, the summed stain at a given grid point could be scaled by the total area represented by that point, which gives the stain value over the range from 0 (meaning no stain area at the given grid point) to 1 (100% of the area at a given grid point is stained). This approach was taken for both the lipid and collagen stains. The identical approach to discretizing the stain and Brillouin maps facilitated their coregistration in the form of pairing the Brillouin shift map to each of the stains, lipid and collagen, respectively, which required only one additional step. Two markers were selected that represented the same points on each of the two images of the shift-stain pairing (identified based on comparable morphology of the sections), which were used to rotate the Brillouin image with respect to the histology image to complete the co-registration. Because the histology and Brillouin images were not identical, only neighbouring, the stiffness and lipid stain area values were averaged circumferentially. This led to 20 values for each metric (Brillouin shift, lipid, and collagen), wherein each value per metric represented each of the 20 equidistantly spaced radial points of the grid. A Spearman’s correlation analysis was then performed to examine the relationship between stain area and Brillouin frequency shift along the radius of each image pairing. Supplemental Figures and Legends Supplemental Figure 1. Diagram of the confocal Brillouin microscope. The laser beam is focused by an oil-immersion objective (100x) into the sample. The scattered light is delivered by a single mode fibre to the 2-stage VIPA spectrometer. The water acts as reference for calibration before measurements. Supplemental Figure 2. Steps used to develop a quantitative spatial map of each stain. (A) Histology images were imported into Clemex and the lumen, IEL, and EEL were manually segmented. (B) A threshold was then applied to the image to identify regions of stain (lipid is shown). (C) The custom script written in Clemex then discretized the stain area (i.e., broke the stain into very small regions or points). The program then outputted the centroid and area (µm2) associated with each stain point, as well as the contour points. (D) The points associated with the lumen and EEL contours (defined now by x-, y- coordinates of each centroid; given by red for EEL and purple for lumen) and stain data (defined by centroids and areas; given by magenta points) were then imported into Matlab. (E) A custom Matlab program was used to create a grid of 30 equidistant points circumferentially and 20 equidistant points radially. The stain areas were then assigned to each point based on proximity and summed. Since each discretized grid point of the vessel cross-section represented a specific area of that cross-section, the summed stain area could be scaled by the total area “represented” by each grid point to address how much of the vessel section in that local region had stain (from 0 – no stain to 1 – 100% of the area was stained). Supplemental References [1] Cheng, C., Tempel, D., van Haperen, R., van der Baan, A., Grosveld, F., Daemen, M.J., Krams, R. & de Crom, R. 2006 Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 113, 2744-2753. (doi:10.1161/CIRCULATIONAHA.105.590018). [2] Brillouin, L. 1922 Diffusion de la lumiere et des rayonnes x par un corps transparent homogene: influence del'agitation thermique. Annals of Physics 17, 88-122. [3] Scarcelli, G., Kim, P. & Yun, S.H. 2011 In vivo measurement of age-related stiffening in the crystalline lens by Brillouin optical microscopy. Biophysical journal 101, 1539-1545. (doi:10.1016/j.bpj.2011.08.008). [4] Shirasaki, M. 1999 Virtually imaged phased array. Fujitsu Sci Tech J 35, 113-125. [5] Scarcelli, G. & Yun, S.H. 2011 Multistage VIPA etalons for high-extinction parallel Brillouin spectroscopy. Optics express 19, 10913-10922. (doi:Doi 10.1364/Oe.19.010913). [6] Antonacci, G., Foreman, M.R., Paterson, C. & Török, P. 2013 Spectral broadening in Brillouin imaging. Appl Phys Lett 103. (doi:Artn 221105 Doi 10.1063/1.4836477). [7] Péry, E., Blondel, W.C.P.M., Thomas, C., Didelon, J., & Guillemin, F. 2006 Diffuse reflectance spectroscopy Monte-Carlo modeling: elongated arterial tissues optical properties, in Modelling and Control in Biomedical Systems 2006 (IPV - IFAC Proceedings Volume), ed. David Dagan Feng, Janan Zaytoon [8] Freek J. van der Meer, Dirk J. Faber, Inci Cilesiz, Martin J.C. van Gemert, Ton G. van Leeuwen Temperature-dependent optical properties of individual vascular wall components measured by optical coherence tomography J. Biomed. Opt. 11(4), 041120 (August 16, 2006). doi: 10.1117/1.2333613