Effect of pH on Biosorption of Basic Dye Malachite Green bt Algae
advertisement

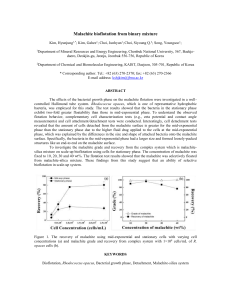
Effect of pH on Biosorption of Basic Dye Malachite Green bt Algae Kawin Pansamrit and Suneerat Ruangsomboon * 1 Program in Fisheries Science, Division of Animal Production Technology and Fisheries, Faculty of Agricultural Technology, King Mongkut’s Institute of Technology Ladkrabang, Bangkok, Thailand Abstract The optimal pH for malachite green (basic dye OT 70701 A) removal from solution by 13 genus of algae (Acanthophora sp., Gracilaria sp., Solieria sp., Padina sp., Turbinaria sp., Dictyota sp., Sargassum sp., Caulerpa lentillifera, Caulerpa sertularioides., Cladophora sp., Ulva rigida, Ulva intestinalis, Chaetomorpha sp., Spirulina platensis and Phormidium angustissimum) was studied. Dried algal cells were added in malachite green solution with initial concentration of 5 mg/L at initial pH 2, 3, 4, 5, 6, 7 and 8 and shaking for 3 h. The optimum pH for malachite green sorption by Solieria sp. was 5, S. platensis., U. intestinalis and U. rigida was 7 and other genus of algae was 6. Padina sp. showed significantly higher biosorption ability, 4.28±0.03 mg/g dry wt., than other algae (p<0.05). Our data indicated that these algae showed high malachite green removal ability at between pH 5-7. Keywords: algae, basic dye, biosorption, malachite green, optimal pH 1. Introduction Treatment of dye eluents presents several problems mainly due to the toxicity and recalcitrance of dyestufs. Discharge of dye eluents into the natural streams may be toxic to the aquatic lives [1]. Color affects the nature of water and inhibits the sunlight penetration into the stream and reduces photosynthetic activity. Some of the dyes are carcinogenic and mutagenic of aquatic ecosystems [2]. Malachite green, basic (cationic) dye, has been extensively used all over the world as a fungicide and ectoparasiticide in aquaculture and very dangerous and has highly cytotoxic property against mammalian cells [3]. The use of biomaterials as sorbents for the treatment of wastewaters will provide as a potential alternate to the conventional treatment and inexpensive ways of removing dyes from large volumes of eluents [1]. Algae are photosynthetic organisms distributed in nearly all parts of the world and in all kinds of habitats [4]. Thus, algae are ubiquitous naturally and serve as one of the biomaterials with high capacity for removing dye from contaminated waters. Marungrueng and Pavasant reported that many functional groups (such as carboxyl, carbonyl, hydroxyl, phosphoryl and amide) making up the algae cell wall played the important roles in dye removal [5]. Several factors play important roles in dye bioremediation. Among these factors, pH, dye concentrations, and amount of biomass of biomaterials are quite important [6]. It’ s has been Corresponding author. Tel-Fax: 66(2)-3298517 E-mail: krsuneer@kmitl.ac.th The 8th International Symposium on Biocontrol and Biotechnology 132 reported that pH not only affects the dye biosorption capacity but also the solubility of dyes [7] and surface binding-sites of cell wall of algae contains several charged group [8]. Therefore pH is an important factor in dye removal. The aim of the present study was to evaluate the effect of pH on malachite green removal from aqueous solution by algae and to evaluate the ability and potential use of algae to remove malachite green. 2. Materials and Methods 2.1 Macroalgae and Microalgae (cyanobacteria) Marine macroalgae were collected from natural and aquatic farm. Caulerpa lentillifera, Caulerpa sertularioides and Acanthophora sp. were collected from Banjong Farm, Chachoengsao province on May. Cladophora sp., Chaetomorpha sp., Ulva rigida and Ulva intestinalis were collected from Trat coastal aquaculture station, Trat province on May. Sargassum sp., Dictyota sp. and Turbinaria sp. were collected from Chonburi province on March. Padina sp. were collected from Trat province. Gracilaria sp. and Solieria sp. were collected from Samutsakron province on April. The algae were washed with water to remove the surface-adhered particles and dried with sun light then powdered to study the malachite green removal ability. Cyanobacteria (Spirulina platensis and Phormidium angustissimum) were obtained from the King Mongkut’s Institute of Technology Ladkrabang, Faculty of Agricultural Technology, Program in Fisheries Science, Division of Animal Production Technology and Fisheries (KMITL, Bangkok, Thailand). S. platensis was cultured in Zarrouk’s medium and P. angustissimum was cultured in BG-11 medium under continuous illumination of 30 µmol photons/m2/s at 28±2ºC. The cyanobacterial cells at 2 weeks period of culture (late exponential phase) were harvested by filtration and dried at 60 ºC. and powdered before studying the malachite green removal ability. 2.2 Basic dye removal ability To study the basic dye removal ability (qeq): 0.01 g (dry weight) of algal cells was added in 10 mL solution of malachite green at concentrations of 5 mg/L at pH 2, 3, 4, 5, 6, 7 and 8 in a 125-mL Erlenmeyer flask. The flasks were shaken at 120 rpm on a shaker at 25 °C for 180 min. Then algal cells were separated by filtration. Malachite green remained in solution was analyzed. The values of qeq were calculated using the mass balance equation: qeq = V(Ci-Ceq)/M where Ci is the initial dye concentration (mg/L), Ceq is the final concentration of dye solution (mg/L), V is the volume of solution (mL), and M is algal dry weight (g). 2.3 Basic dye and Statistical analysis Basic dye (malachite green) concentration was analyzed by a spectrophotometer (Becthai, Thailand) at wavelength 618 nm. All experiments were conducted in three replicates. Significant differences were determined using analysis of variance (ANOVA) with 95% confidence (probability limit of p<0.05). 3. Results and Discussion Malachite green removal ability of algae with various pH of solution was shown in Table 1 and Figure 1. The optimum pH for malachite green removal by Solieria sp. was 5, S. platensis, U. intestinalis and U. rigida was 7 and other genus of algae was 6. At their optimum pH, Padina sp. showed significantly higher removal ability, 4.28±0.03 mg/g dry wt., than other algae (p<0.05). The 8th International Symposium on Biocontrol and Biotechnology 133 Solieria sp. (0.50±0.03 mg/g dry wt.) and Chaetomorpha sp. (0.61±0.02 mg/g dry wt.) showed significantly lower dye removal ability than other algae (p<0.05). This study indicates that the aqueous solution pH exerts profound influence on the sorptive uptake of dyes presumably due to its impact on both the surface binding-sites of cell wall of algae which contains different functional groups such as carboxyl, hydroxyl, sulphate and other charged group and the ionization or aggregation process of the dye molecules [8]. This can be explained on the basis of zero point charge for algal biomass that the isoelectric point would be pH 3.0 [1, 4]. Table 1. Basic dye removal ability ( mg/g dry wt.) of algae with various pH (initial dye concentration of 5 mg/l) pH Algae 2 3 4 5 6 7 8 Red algae Acanthophora sp. 0.47±0.01cA 0.82±0.02cB 1.32±0.02eC 1.74±0.01cD 2.29±0.03eF 2.10±0.01cE 2.08±0.02cE Gracilaria sp. 1.45±0.02fA 1.67±0.02eB 2.69±0.06hC 2.71±0.01jC 3.32±0.01fD 3.25±0.01gD Solieria sp. Brown algae 0.22±0.03bA 0.32±0.05aB 0.39±0.01aBC 0.50±0.03abD 0.49±0.02aD 0.47±0.01aCD 0.45±0.01aCD Padina sp. 2.15±0.03iA 2.30±0.04fgB 2.60±0.02hC 2.65±0.01iC 4.28±0.03kF 3.43±0.02fghD 3.57±0.02hiE Turbinaria sp. 2.43±0.05jA 2.77±0.04hB 3.01±0.02iC 3.10±0.02lC 4.05±0.03jE 3.41±0.02fgD 3.49±0.02hiD iA 3.48±0.02ghiE 3.48±0.01hE 1.35±0.06eA 1.51±0.09dB 2.90±0.02iC 2.91±0.02kC 4.10±0.02jE 3.60±0.01iD 3.63±0.01iD Caulerpa sertularioides 1.68±0.04hA 1.78±0.07eA 2.54±0.03hB 2.57±0.01hB 3.17±0.03gD 2.68±0.01eC 2.76±0.02fC Caulerpa lentillifera 0.43±0.03cA 0.92±0.01cB 2.14±0.02fC 2.13±0.01gC 2.32±0.02eE 2.23±0.00cD 2.18±0.03cdCD Cladophora sp. 1.54±0.02gA 2.35±0.02gB 2.66±0.05hC 2.71±0.01jC 3.58±0.00hiD 3.53±0.05hiD 3.90±0.04iE 0.72±0.02bC 0.71±0.01bC 0.82±0.01bC 0.75±0.03bC 0.33±0.02aB 0.54±0.02bC 0.54±0.02bC 0.61±0.02abD 0.52±0.01aC 0.54±0.02aC 0.56±0.01dA 0.83±0.04cA 1.40±0.16eB 1.48±0.01eB 2.41±0.16eD 0.41±0.05cA 0.90±0.02cB 1.07±0.03dC 1.39±0.03dD 2.55±0.04fF 0.34±0.02 0.47±0.01 0.47±0.01 aC 3.81±0.01 hiF Sargassum sp. Green algae abC 3.07±0.03 lD 2.11±0.01 aB 2.35±0.01 gC Dictyota sp. aA 2.22±0.01 fB 3.75±0.04hE Ulva rigida 0.1±0.02 Ulva intestinalis 0.16±0.04abA 0.51±0.06bA 0.73±0.02cB 0.75±0.02cB Chaetomorpha sp. Cyanobacteria 0.10±0.01aA Spirulina platensis Phormidium angustissimum 0.72±0.07 bcD 0.78±0.01cE 2.00±0.14dC 2.44±0.10dD 2.21±0.01cE 2.23±0.02dE The same small letter in each row mean absence of statistical differences (p>0.05) The same capital letter in each column mean absence of statistical differences (p>0.05) At a higher pH above this zero point charge, the surface of biomass gets negatively charged, which enhances the positively charged of dye cations through electrostatic force of attraction and at lower pH, the surface charge may get positively charged (i.e. protonation of the cell wall), thus making (H+) ions compete effectively with dye cations toward actives sorption sites causing a decrease in the amount of adsorbed dye [1, 4, 8]. Many studies have been done on effect of initial pH solution on the color removal effciency of malachite green solution by green algae. Cosmarium sp. Malachite green removed ability was analyzed over a pH range from 2.0 to 11.0. It shown that an increase in pH from 4.0 to 6.0 leads to a threefold increase in decolorization The 8th International Symposium on Biocontrol and Biotechnology 134 rate, which is reached the maximum value of 92.4% at the pH of 9.0 and the color removal effciency from 26.3% to 68.4% for an increase in pH from 3.0 to 4.0 [4]. i h g h g g f de d d bc a c e ab Figure 1. Basic dye removal ability (qeq, mg/g dry wt.) of algae with optimal pH (initial dye concentration of 5 mg/l). The same small letter mean absence of statistical differences (p>0.05) The influence of initial dye solution pH of malachite green removal by a fresh water algae Pithophora sp. at different solution pH ranging from 2 to 7 at initial malachite green concentration 50 mg/L was studied. It was shown that maximum of 64.4 mg/g of dye was adsorbed at a pH of 6 [1]. The optimum initial pH for malachite green removal by brown algae Turbinaria conoides biomass at initial dye concentration 100 mg/L was found to be 8.0 and showed maximum adsorption capacity 66.6 mg/g [9]. The effect of pH on removal of basic dye, methylene blue using green alga Enteromorpha spp. was investigated for values between 2 and 10. The sorption capacity was minimum at pH 2 (40.21 mg/g) and increased up to pH 6, then remained nearly constant (70.35 mg/g) over the initial pH ranges of 6–10 [10]. The percentage sorption of methylene blue by giant duckweed Spirodela polyrrhiza at initial pH between 2-11 was reported. S. polyrrhiza showed minimum sorption at the initial pH 2, and the percentage sorption of methylene blue was not significantly altered (p>0.05) when the initial pH was increased from 3 to 11. S. polyrrhiza showed maximum adsorption capacity between 126.58-129.87 mg/g [11]. 4. Conclusions The optimal pH for malachite green ( basic dye OT 70701 A) removal by Soliera sp. was 5, Spirulina platensis, Ulva intestinalis and Ulva. rigida was 7 and Acanthophora sp., Gracilaria sp., Padina sp., Turbinaria sp., Dictyota sp., Sargassum sp., Caulerpa lentilifera, Caulerpa The 8th International Symposium on Biocontrol and Biotechnology 135 sertularioides, Cladophora sp., Chaetomorpha sp. and Phormidium angustissimum was 6. Padina sp. showed the highest malachite green removal ability. 5. Acknowledgements This study was grants by the National Research Council of Thailand. References [1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] Kumar, K.V., Ramamurthi, V. and Sivanesan S., 2006. Biosorption of malachite green, a cationic dye onto Pithophora sp., a fresh water algae. Dyes and Pigments, 69, 102-107. Sivaraj, R., Namasivayam, C. and Kadirvelu, K., 2001. Orange peel as an adsorbent in the removal of acid violet 17 (acid ye) from aqueous solutions, Waste Management, 21, 105-10. Bekci, Z., Seki, Y. and Cavasb, L., 2009. Removal of malachite green by using an invasive marine alga Caulerpa racemosa var. cylindracea. Journal of Hazardous Materials, 161, 1454–1460. Daneshvar, N., Ayazloo M., Khataee, A.R. and Pourhassan, M., 2007. Biological decolorization of dye solution containing Malachite Green by microalgae Cosmarium sp. Bioresource Technology, 98, 1176–1182. Marungrueng, K. and Pavasant, P., 2007. High performance biosorpbent ( Caulerpa lentillifera) for basic dye removal. Bioresource Technology, 98, 1567-1572. Wafaa, M., El-Rahim, A. and El-Ardy, O.A.M., 2008. Hassan Moawad, Aeration as a factor in textile dye bioremoval by Aspergillus niger. African Journal of Biochemistry Research, 2(1), 30–39. Fu, Y.Z. and Viraraghavan, T., 2001. Removal of CI Acid Blue 29 from an aqueous solution by Aspergillus niger. American Association of Textile Chemists and Colorists Review, 1(1), 36–40. Tsai W.T. and Chen H.R., 2010. Removal of malachite green from aqueous solution using low-cost chlorella-based biomass. Journal of Hazardous Materials, 175, 844–849. Kannan, R.R., Rajasimman, M., Rajamohan, N. and Siaprakash, B., 2010. Brown marine algae Turbinaria conoides as biosorbent for Malachite green removal: Equilibrium and kinetic modeling. Frontiers of Environmental Science and Engineering in China, 4(1), 116– 122. Ncibi, M.C., Hamissa, A.M.B., Fathallah, A., Kortas, M.H., Baklouti, T., Mahjoub, B. and Seffen, M., 2009. Biosorptive uptake of methylene blue using Mediterranean green alga Enteromorpha spp. Journal of Hazardous Materials, 170, 1050–1055. Waranusantigul, P., Pokethitiyooka, P., Kruatrachuea, M. and Upathama, E.S., 2003. Kinetics of basic dye (methylene blue) biosorption by giant duckweed (Spirodela polyrrhiza). Environmental Pollution, 125, 385–392. The 8th International Symposium on Biocontrol and Biotechnology 136