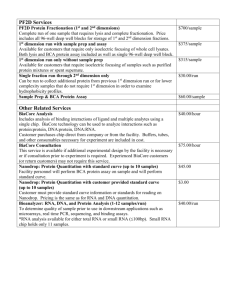
Supplementary Information (doc 44K)

SUPPLEMENTAL INFORMATION
Splenocyte stimulation
Splenocytes were isolated from spleens which were mechanically broken up and passed through 70μm Nylon Cell Strainers to isolate a single cell suspension. Red blood cells were removed following the procedure detailed in the Mouse Erythrocyte
Lysing Kit (R+D Systems, Abingdon, UK). Isolated cells were washed twice, counted through trypan blue exclusion and re-suspended to a concentration of 2x10 6 cells/ml in DMEM supplemented with 10% foetal bovine serum (FBS) and 1% penicillin/streptomycin antibiotics. Duplicate wells remained unstimulated or were stimulated with 1μg/ml lipopolysaccharide (LPS) (Sigma, Dublin, Ireland) and incubated at 37ºC in 5% CO2 for 48 hours. Plates were then centrifuged at 300g and supernatants aspirated and stored at for analysis. Levels of TNF were determined by cytometric bead array (BD, Oxford, UK) using a BD FACSCalibur flow cytometer according to the manufacturer’s instructions.
Serotonin and 5-HIAA HPLC assay
Neurotransmitter concentrations were determined using a modification of a previously described procedure
1 . Briefly, brain tissue was sonicated in 500μl of chilled mobile phase spiked with 4ng/40ul of N-Methyl 5-HT (Sigma, UK) as internal standard. The mobile phase contained 0.1M citric acid, 0.1M sodium dihydrogen phosphate,
0.01mM EDTA (Alkem/Reagecon, Cork, Ireland), 5.6mM octane-1-sulphonic acid
(Sigma) and 9% (v/v) methanol (Alkem/Reagecon), and was adjusted to pH 2.8 using
4N sodium hydroxide (Alkem/Reagecon). Homogenates were then centrifuged at
21,000g for 20 minutes at 4 o C and 40μl of the supernatant injected onto the HPLC system (Electrochemical detection). A reverse-phase column (Kinetex 2.6u C18 100 x
4.6mm, Phenomenex, UK) maintained at 30 o
C was employed in the separation (Flow rate 0.9ml/min), the glassy carbon working electrode combined with an Ag/AgCL reference electrode (Shimdazu) was operated a +0.8V and the chromatograms generated were analysed using Class-VP 5 software (Shimadzu). Serotonin (5-HT) and its main metabolite 5-HIAA were identified by their characteristic retention times as determined by standard injections which were run at regular intervals during the sample analysis. Analyte:Internal standard peak height rations were measured and compared with standard injections and results were expressed as ng of neurotransmitter per g fresh weight of tissue.
Tryptophan and kynurenine pathway metabolites HPLC assay
Tryptophan and kynurenine pathway metabolites were determined as previously described 2 . Briefly, plasma samples were spiked with internal standard (3-Nitro ltyrosine) prior to being deproteinised by the addition of 20 μl of 4M perchloric acid to
200 μl of sample. Samples were centrifuged at 21000g on a Hettich Mikro 22R centrifuge (AGB, Dublin, Ireland) for 20 minutes at 4°C and 100 μl of supernatant transferred to a HPLC vial for analysis on the HPLC system (UV and FLD detection).
All samples were injected onto a reversed phase Luna 3 μm C18 (2) 150 × 2 mm column (Phenomenex), which was protected by Krudkatcher disposable pre-column filters (Phenomenex) and SecurityGuard cartridges (Phenomenex). The mobile phase consisted of 50 mM acetic acid, 100 mM zinc acetate with 3% (v/v) acetonitrile and was filtered through Millipore 0.45 μm HV Durapore membrane filters (AGB) and vacuum degassed prior to use. Compounds were eluted isocratically over a 30-minute runtime at a flow rate of 0.3 mls/min after a 20 μl injection. The column was maintained at a temperature of 30°C and samples/standards were kept at 8°C in the cooled autoinjector prior to injection. The fluorescent detector was set at an excitation
wavelength of 254 nm and an emission wavelength of 404 nm. The UV detector was set to 330 nm. L-tryptophan and its metabolites (kynurenine, kynurenic acid) were identified and quantified as described above for 5-HT and results were expressed as ng/ml of plasma. qRT-PCR Analysis
Total RNA was isolated using the Stratagene Absolutely RNA miniprep kit (Agilent).
RNA was then treated with turbo DNAase (Ambion/Applied Biosystems) to ensure
DNA free samples and stored at −80 °C until analysis. RNA concentration/quality was determined using the standard OD260/280 method using a Nanodrop spectrophotometer (Mason Technology, Cork, Ireland). The OD260/OD280 ratio for each RNA sample used in subsequent experiments was in the range 1.9-2.1. The quality of the RNA was further confirmed using the Bioanalyser (Agilent, Dublin,
Ireland) and only samples with a RIN number of >7 were included in the analysis.
RNA was converted into cDNA using the high capacity cDNA archive kit (Applied
Biosystems, Warrington, UK). Taqman assays (Applied Biosystems) were used in all experiments and with the exception of Bdnf, all targets were available as off the shelf assays. The Taqman assay for Bdnf was custom made using the Applied Biosystems
File Builder software (version 3.1) and was designed, based on the previously identified sequence, to amplify all variants of the Bdnf gene. The targets and assay
IDs were as follows: Htr1a (Mm00434106_s1); Htr2c (Mm00434127_m1); Htr6
(Mm00445320_m1); Slc6a4 (Mm00439391_m1); Tph2 (Mm00557717_m1); Bdnf
( AIGI3YX, all variants). All assays were run in triplicate with β-actin as housekeeping gene (Assay ID Mm00607939_s1) under standard conditions on the
Applied Biosystems Real Time PCR 7300 system. All data were analysed using the
2
ΔΔCt
relative quantitation method
3
and expressed as fold change compared to control values.
Corticosterone Assay
Plasma corticosterone levels were measured using a Corticosterone Immunoassay Kit according to the manufacturers instructions (Assay Designs, Ann Arbor, MI, USA).
The assay has a sensitivity of 26.99 pg/ml.
Light-Dark box
Mice were assessed for anxiety in the light-dark box as previously described
4
. The apparatus consisted of a plexiglas enclosure (44cm×21cm×21cm, L×W×H) separated into two compartments, a large and brightly lit open area (transparent), and a smaller, dark closed and safe part (14cm long), comprising a small opening (10cm×5cm) to allow transitions between the two parts. Mice were individually placed into the light part, and allowed 10-min free exploration. The number of transitions between the two parts were manually scored post test using the recorded videos. At the end of the trial, mice were placed back into their home cage with littermates.
Referances
1. O'Mahony S, Chua AS, Quigley EM, Clarke G, Shanahan F, Keeling PW et al.
Evidence of an enhanced central 5HT response in irritable bowel syndrome and in the rat maternal separation model. Neurogastroenterol Motil 2008;
20 (6) : 680-688.
2. Clarke G, Fitzgerald P, Cryan JF, Cassidy EM, Quigley EM, Dinan TG.
Tryptophan degradation in irritable bowel syndrome: evidence of indoleamine
2,3-dioxygenase activation in a male cohort. BMC Gastroenterol 2009; 9: 6.
3. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods
2001; 25 (4) : 402-408.
4. O'Mahony CM, Sweeney FF, Daly E, Dinan TG, Cryan JF. Restraint stressinduced brain activation patterns in two strains of mice differing in their anxiety behaviour. Behav Brain Res 2010; 213 (2) : 148-154.