1. Upper Airway Physiology in Health and Disease.
advertisement

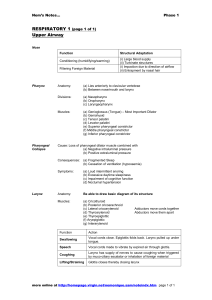
RESPIRATORY FUNCTION OF THE UPPER AIRWAYS – FROM PHYLOGENY AND ONTOGENY TO PHYSIOLOGY Jean-Paul Praud Departments of Pediatrics and Physiology Université de Sherbrooke Corresponding author: Jean-Paul Praud MD PhD Tel: (819) 346-1110, ext. 14851 Departments of Pediatrics and Physiology Fax: (819) 564-5215 Université de Sherbrooke email: Jean-Paul.Praud@USherbrooke.ca J1H 5N4, QC Canada Acknowledgments Jean-Paul Praud is the holder of the Canada Research Chair in Neonatal Respiratory Physiology. His research is supported by the Canadian Institutes for Health Research, the Fonds de la recherche en santé du Québec and the Foundation of Stars. INTRODUCTION The upper airways exert an important influence on breathing. In addition to participating in fetal lung growth, in the successful transition towards air breathing at birth and in the maintenance of optimal lung ventilation thereafter, the larynx is also involved in swallowing and protection of the lower airways. Furthermore, neural immaturity in the newborn is often responsible for reflexes originating from the laryngeal region, the laryngeal chemoreflexes, which are inhibitory to cardiorespiratory function. This short review will use phylogenetic and ontogenetic background to introduce a few aspects of postnatal upper airway function related to apneas, laryngeal chemoreflexes and swallowing-breathing coordination. PHYLOGENY: UPPER AIRWAYS AND RESPIRATION A pharyngeal pump and a laryngeal closing valve for initial air-breathing in vertebrates Early lungfishes acquired the ability to use environmental air to fulfill their metabolic requirements more than 370 million years ago. As bimodal breathers, they were capable for the first time of ventilating a primitive lung intermittently, in addition to water breathing. The simultaneous appearance of a closing valve, the primitive larynx, was critical to this evolutionary step to protect the lungs from flooding during feeding and water breathing. Amphibians were the first to become dependent on air breathing. Air breathing was accomplished by filling the oral cavity with air through the nares by passive recoil of the pharyngeal wall, then forcing air into the lungs by pharyngeal muscle contraction and holding air in the lungs by laryngeal closure, much as the lungfish. With evolution, modern amphibians acquired a well-developed larynx homologous to those of higher vertebrates, with a cartilage skeleton and strong, paired dilator muscles in addition to muscles that close the glottal aperture (1). Disappearance of pharyngeal pump mechanism for breathing with vertebrate evolution A significant problem with the pharyngeal pump mechanism of filling the lungs is that tidal volume is constrained by the size of the pharynx. Evolution towards vertebrates with large bodies and small heads such as reptiles was only made possible by adding a thoracic aspiratory system, i.e., inspiratory thoracic muscles. Contraction of the latter, especially the diaphragm, has remained of crucial importance for lung breathing in mammals. Still, while the pharyngeal contraction phase has disappeared from the breathing cycle, active laryngeal closure remains prominent in today’s vertebrates in certain conditions. Indeed, though absent in most adult terrestrial mammals, active post-inspiratory laryngeal closure remains a basic component of the breathing cycle in many lower vertebrates and in diving mammals, even on land (2). In addition, active post-inspiratory laryngeal closure represents a mechanism of major importance for early postnatal breathing in terrestrial mammals (see below). ONTOGENY: FROM FLUID-FILLED AIRWAYS TO AERIAL BREATHING While the nasal airway originates from invagination of the ectoderm, the skeleton and muscles of the mouth, pharynx and larynx develop from pharyngeal arches and clefts in the embryo. Following anatomical and functional development, the pharynx and larynx are actively engaged in swallowing and breathing movements in the fetus. Respiratory function of the larynx in fetal life In the fetal mammal, the larynx is actively closed when fetal breathing movements are absent, reminiscent of the lungfish during diving. Fetal lung growth relies heavily on the high pressure present in the liquid-filled airways generated by this glottal closure, which opposes continuous secretion of lung liquid by the airway epithelium. In addition, coordinated contraction of pharyngeal/laryngeal dilator muscles and diaphragm is observed during bursts of fetal breathing movements (3). However, these fetal breathing movements do not entrain amniotic liquid into the trachea. Indeed, when necessary, laryngeal constrictor muscles contract to defend the entrance of the trachea against influx of amniotic fluid filled with debris (lanugo, vernix caseosa) via reflex glottal closure. Such laryngeal chemoreflexes are due to laryngeal receptors sensitive to the lower chloride concentration of the amniotic fluid (4). Finally, breathing-swallowing coordination develops in the fetus, allowing oral feeding around 35 weeks of gestation in the newborn infant born prematurely. Respiratory function of the upper airways at birth and in the early postnatal period At birth, complete, active glottal closure throughout the very first expirations is vital for establishing an end-expiratory lung volume of air, i.e. the initial functional residual capacity. In the first hours and days after birth, an active post-inspiratory laryngeal closure is frequently observed. By decreasing lung emptying, this expiratory airflow braking mechanism defends functional residual capacity against low lung compliance present at that age (5). The muscular pharyngeal tube, which is so important for glossopharyngeal respiration in amphibians, however does not retain any respiratory advantage in mammals after birth. On the contrary, its collapsible characteristics render phasic contraction of pharyngeal dilator muscles necessary just before diaphragm inspiratory contraction to prevent unwanted pharyngeal narrowing secondary to decreased intraluminal pressure (6). Upper airways and apneas Premature infants born before 27 weeks are virtually all affected by apneas of prematurity, which are responsible for bradycardia and desaturation and carry the potential of neurological sequelae. Complete, active laryngeal closure during central apneas in the newborn. Our group has shown that complete, active glottal closure with maintenance of a high apneic lung volume throughout apneas was consistently noted during periodic breathing (7), as well as during most post-sigh apneas in preterm lambs. Such observations were concordant with previous reports in dog pups, newborn opossums and human newborns. Maintenance of a high lung volume during central apneas increases alveolar O2 stores and limits post-apneic arterial O2 desaturation (8). Such active inspiratory breath-holding has phylogenetic and ontogenetic correlations (see above) and is not related to reflexes originating from laryngeal receptors, e.g., laryngeal chemoreflexes. Of note, while prominent in newborns, closure of the laryngeal valve during central apneas has also been observed in adult humans. Passive pharyngeal collapse during central apneas. Loss of central respiratory drive induces passive pharyngeal narrowing during central apneas. Insufficient pharyngeal dilator muscle contraction at breathing resumption can cause pharyngeal closure, i.e., a mixed apnea. The latter is frequently seen in newborns, as well as in older children and adults with obstructive sleep disordered breathing. In fact, pharyngeal lumen size during breathing results from interaction between anatomical and neural mechanisms. Any anatomical imbalance between soft tissue volume (increased with macroglossia, peripharyngeal fat pads, adenotonsillar hypertrophy, …) and bony enclosure size (decreased with microretrognathia, syndromic malformations, orthodontic anomalies, …) favors pharyngeal closure. In addition, any neural imbalance between the collapsing force of inspiratory thoracic muscle contraction and dilating force of pharyngeal dilator muscles (decreased by prematurity, REM sleep, sedation, …) favors pharyngeal closure (9). Laryngeal chemoreflexes Laryngeal chemoreflexes represent another prominent manifestation of the original, protective valve function of the vertebrate larynx. Laryngeal chemoreflexes (LCR) are triggered by the contact between liquids - especially acid or with low chloride content - and receptors of the laryngeal mucosa in mammals. These lung protective reflexes consist primarily of swallowing, coughing and arousal in mature mammals, thus limiting larynx penetration and tracheal aspiration (4). However, in the immature, newborn mammal, LCR are composed of both a vagal component, which includes laryngospasm, apnea, oxygen desaturation and bradycardia, and a sympathetic component, which includes systemic hypertension and redistribution of blood flow to vital organs (4). Clinical relevance of LCR stems from the observation that they are often triggered by gastric reflux, bottle-feeding or oral intake of liquid medications in preterms. In addition, they can be responsible for apparent life-threatening events and probably some cases of sudden infant death syndrome (10). Abnormal conditions, such as respiratory syncytial virus infection in young infants or laryngopharyngeal reflux during sleep in older children, exacerbate the potentially dangerous cardiorespiratory components of the LCR. SWALLOWING AND BREATHING ACTIVITY: THE DANGEROUS LIAISONS The pharynx was involved in feeding long before the emergence of air breathing in lungfishes. With air breathing, however, breathing and swallowing became competing functions at the pharyngeal level. Swallowing activity involves the coordinated contraction of more than 25 pairs of upper airway muscles in a sequence designed and coordinated by the brainstem swallowing center. In addition, a precise swallowing-breathing coordination, which includes an obligatory respiratory pause, is a necessity to prevent both prolonged apnea and tracheal aspiration (11). Thus, while forceful contraction of pharyngeal constrictor muscles propels the food bolus into the esophagus, the tensor and elevator veli palatini and the laryngeal constrictor muscles contract to prevent entry of food into the nasopharynx and the trachea respectively. In humans, phonation modifies upper airway anatomy and adds further complexity to breathing-swallowing interaction (1). In the newborn, before development of phonation, the larynx is cephalad, and the overlapping epiglottis and soft palate establish a nasal airway for respiration during milk swallowing. This anatomical configuration explains that human infants are preferential nose breathers for the first 6 weeks to 6 months. Postnatal modifications of the upper airways to allow sound production lead to descent of the larynx in the neck and loss of epiglottis-soft palate contact. Increased risks of tracheal aspiration are then prevented by elevation of the larynx during swallowing, by the shape of the epiglottis, which directs food laterally into the pyriform fossae, and by the aryepiglottic folds + arytenoids, which act as ramparts to prevent laryngeal penetration. In the meantime, maturation of the swallowing and respiratory centers in the brainstem ensures an optimal swallowing-breathing coordination. Conversely, immaturity, neuromuscular disorders, laryngeal inflammation or upper airways malformations, for example, can be responsible for inadequate swallowing-breathing coordination and tracheal aspiration in children. REFERENCES 1. Bartlett D Jr. Upper Airway Motor Systems. Comprehensive Physiology 2010; 223–245. 2. Shelton G, Jones DR, Milsom WK. Control of Breathing in Ectothermic Vertebrates. Comprehensive Physiology 2010; 857–909. 3. Kianicka I, Diaz V, Dorion D, Praud J-P. Coordination between glottic adductor muscle and diaphragm EMG activity in fetal lambs in utero. J Appl Physiol 1998; 84:1560-1565. 4. Thach BT. Some aspects of clinical relevance in the maturation of respiratory control in infants. J Appl Physiol 2008; 104:1828-1834. 5. Mortola JP. Dynamics of breathing in newborn mammals. Physiol Rev. 1987; 67:187-243. 6. Milner AD, Greenough A. The role of the upper airway in neonatal apnoea. Semin Neonatol 2004; 9:213-219. 7. Renolleau S, Letourneau P, Niyonsenga T, Praud J-P. Thyroarytenoid muscle electrical activity during spontaneous apneas in preterm lambs. Am J Respir Crit Care Med 1999; 159:1396-1404. 8. Reix P, Arsenault J, Dome V, Fortier PH, Lafond JR, Moreau-Bussiere F et al. Active glottal closure during central apneas limits oxygen desaturation in premature lambs. J Appl Physiol 2003; 94:1949-1954. 9. Tsuiki S, Isono S, Ishikawa T, Yamashiro Y, Tatsumi K, Nishino T. Anatomical balance of the upper airway and obstructive sleep apnea. Anesthesiology 2008; 108:1009-1015. 10. Leiter JC, Böhm I. Mechanisms of pathogenesis in the Sudden Infant Death Syndrome. Respir Physiol Neurobiol 2007; 159:127-138. 11. Praud J-P, Reix P. Upper airways and neonatal respiration. Respir Physiol Neurobiol 2005; 149:131-141.