Unit 3: September 16
advertisement
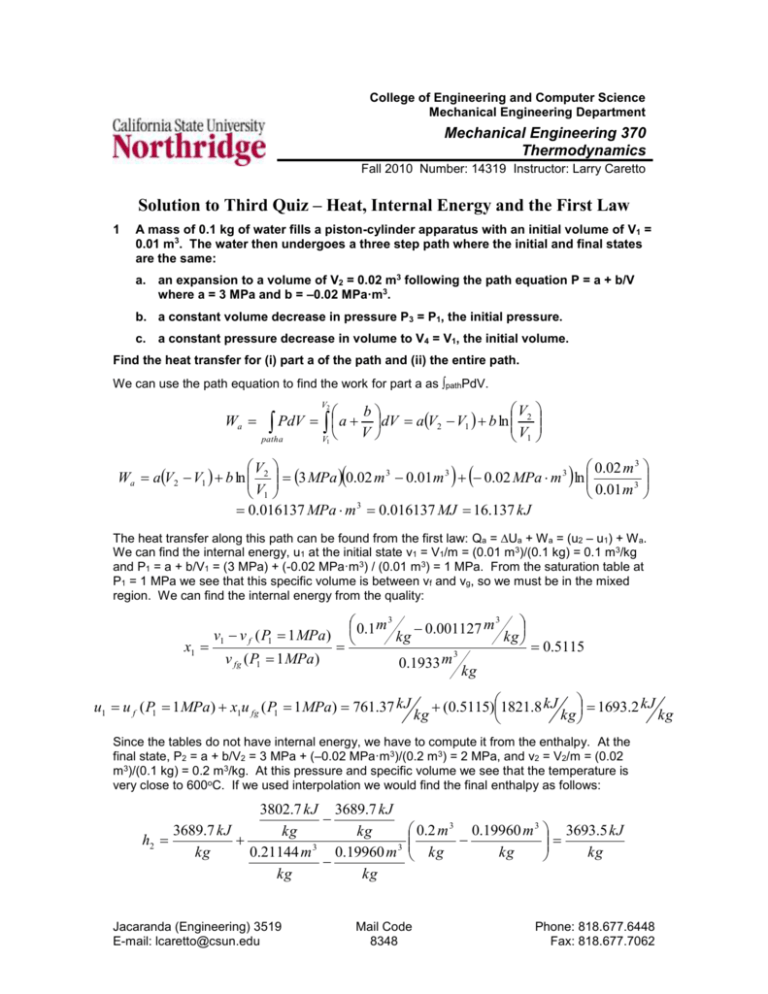
College of Engineering and Computer Science Mechanical Engineering Department Mechanical Engineering 370 Thermodynamics Fall 2010 Number: 14319 Instructor: Larry Caretto Solution to Third Quiz – Heat, Internal Energy and the First Law 1 A mass of 0.1 kg of water fills a piston-cylinder apparatus with an initial volume of V1 = 0.01 m3. The water then undergoes a three step path where the initial and final states are the same: a. an expansion to a volume of V2 = 0.02 m3 following the path equation P = a + b/V where a = 3 MPa and b = –0.02 MPa·m3. b. a constant volume decrease in pressure P3 = P1, the initial pressure. c. a constant pressure decrease in volume to V4 = V1, the initial volume. Find the heat transfer for (i) part a of the path and (ii) the entire path. We can use the path equation to find the work for part a as pathPdV. 2 V b PdV a dV aV2 V1 b ln 2 V V1 path a V1 V Wa 0.02 m 3 V2 3 3 3 Wa aV2 V1 b ln 3 MPa 0.02 m 0.01 m 0.02 MPa m ln 3 V1 0.01 m 0.016137 MPa m 3 0.016137 MJ 16.137 kJ The heat transfer along this path can be found from the first law: Qa = Ua + W a = (u2 – u1) + W a. We can find the internal energy, u1 at the initial state v1 = V1/m = (0.01 m3)/(0.1 kg) = 0.1 m3/kg and P1 = a + b/V1 = (3 MPa) + (-0.02 MPa·m3) / (0.01 m3) = 1 MPa. From the saturation table at P1 = 1 MPa we see that this specific volume is between vf and vg, so we must be in the mixed region. We can find the internal energy from the quality: x1 v1 v f ( P1 1 MPa) v fg ( P1 1 MPa) 0.1 m 3 0.001127 m 3 kg kg 0.5115 3 m 0.1933 kg u1 u f ( P1 1 MPa) x1u fg ( P1 1 MPa) 761.37 kJ kg (0.5115)1821.8 kJ 1693.2 kJ kg kg Since the tables do not have internal energy, we have to compute it from the enthalpy. At the final state, P2 = a + b/V2 = 3 MPa + (–0.02 MPa·m3)/(0.2 m3) = 2 MPa, and v2 = V2/m = (0.02 m3)/(0.1 kg) = 0.2 m3/kg. At this pressure and specific volume we see that the temperature is very close to 600oC. If we used interpolation we would find the final enthalpy as follows: 3802.7 kJ 3689.7 kJ 3689.7 kJ kg kg h2 3 kg 0.21144 m 0.19960 m 3 kg kg Jacaranda (Engineering) 3519 E-mail: lcaretto@csun.edu 0.2 m 3 0.19960 m 3 3693.5 kJ kg kg kg Mail Code 8348 Phone: 818.677.6448 Fax: 818.677.7062 Solutions to third quiz ME 370, L. S. Caretto, Fall 2010 Page 2 We can now find the internal energy at the end of the first path, u2. u 2 h2 P2 v2 3689.7 kJ 0.2 m 3 1000 kJ 3293.5 kJ 2 MPa 3 kg kg MPa m kg We can now find the heat transfer for step a from the first law. 3293.5 kJ 1693.2 kJ 16.137 kJ Qa mu 2 u1 Wa 0.1 kg kg kg Qa = 176.2 kJ For the entire path the initial and final states are the same so the internal energy change is zero. (U depends only on the state; it the initial and final states are the same, the value of u at both states is the same so DU = 0) This gives Q = W = W a + W b + W c for the overall path. We have found W a = 16.137 kJ and we know that W b = 0 for the constant volume step in part b of the path. The third part of the path, at constant pressure P3 = P4 = P1 = 1 MPa is found as follows: W c = Pconst (V4 – V3) = (1 MPa)(0.01 m 3 – 0.02 m3) = –0.01 MPa·m3 = –0.01 MJ = –10 kJ. The heat transfer for the overall path is then found to be Q = W = W a + W b + W c = 16.137 kJ + 0 + (–10 kJ) or Q = 6.137 kJ








