Utilization of NiO nanowires in dye
advertisement

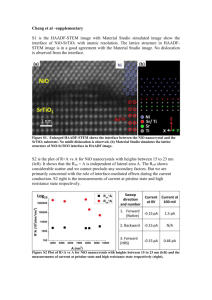
Utilization of NiO nanowires in dye-sensitized solar cells Shuo Liu Upper Arlington High School February, 2013 Utilization of NiO nanowires in dye-sensitized solar cells Shuo Liu, 2817 Welsford Road, Upper Arlington, Ohio 43221 Upper Arlington High School, Upper Arlington, Ohio 43221 Mentor: Yiying Wu The purpose of this study was to utilize NiO nanowires in dye-sensitized solar cells (DSSCs) and compare its efficiency with that of a common NiO dye-sensitized solar cell. A sea-urchin like morphology that consists of arrays of nanowires was synthesized by a hydrothermal reaction procedure. Films made from both normal and sea-urchin NiO particles have been used to fabricate DSSCs. The efficiencies of both cells were calculated after data collection and the augmented film with the sea-urchin particles was more efficient. This could lead to further improvements of the efficiency of dyesensitized solar cells through the use of different substrate morphologies. Improving the efficiency of these solar cells allows it to be a viable alternative source of photovoltaic energy. ACKNOWLEDGEMENTS First and foremost I would like to thank Mrs. Laura Brennan, the Science Research coordinator at Upper Arlington High School for making this all possible and helping me through the research project. I would also like to thank my mentor, Dr. Yiying Wu, a professor of chemistry at Ohio State University who allowed me to conduct research in his labs. My thanks also goes out to Thomas Draskovic who helped me out with my experiments in the lab. Last but not least, I would like to thank my parents for supporting and encouraging me all the way through my project. TABLE OF CONTENTS List of Tables……………………………………………………………………………...1 List of Figures……………………………………………………………………………..1 Introduction………………………………………………………………………………..2 Methodology………...…………………………………………………………………….4 Results……………………………………………………………………………………..7 Discussion and Conclusion..………………………………………………………..……10 References………………………………………………………………………………..11 Appendices……………………………………………………………………………….12 LIST OF TABLES TABLE 1 O3 Dye-Sensitized Solar Cell Comparison………………………………7 LIST OF FIGURES FIGURE 1 Sea-urchin Particle under SEM…………………………………………7 FIGURE 2 O3 Dye-Sensitized Solar Cell Graph……………………………………8 INTRODUCTION In today’s era, the world is suffering through an energy crisis. Fossil fuels, the main energy supply of the world for almost two centuries, are beginning to dwindle. Some estimates predict fossil fuels supplies might even run out in 100 years, leaving our future generations with nothing. Also, compounded with the problem of the disappearing fuel sources is the pollution these fuel sources cause. Burning coal and other fossil fuels releases harmful chemicals into the air, making living conditions for some people less than ideal. Using coal also causes the emission of CO2 into the atmosphere, adding on to the green house effect and slowly destroying the natural environment. New solutions to the energy crisis are being invented, primarily based upon three alternative forms of energy: solar, wind, and water. All three of these sources provide clean, renewable energy, which effectively eliminates the problem caused by fossil fuels. Solar energy is based off of the concept of using the sun as a source of energy. Usually, the sun’s light is converted into electricity by devices known as solar cells. The photovoltaic industry today is still dwarfed by the fossil fuel companies, but is developing into a realistic alternative to these fossil fuels. Some problems with traditional solar cells, however, are that they are costly to manufacture and are inefficient. A newer approach to photovoltaic energy is dye-sensitized solar cells. They are based off of the ideas of the traditional solar cell, but they utilize dyes instead of expensive semiconductors. This branch of solar power is interesting, but like the others, it suffers in its efficiency. Because the energy crisis is only going to worsen as time progresses, new innovative solutions must be found. In the field of photovoltaic energy, none of the solar cells have combined good efficiency with low cost and maintenance. A solution to this problem will be hard to find, but may be found in the branch of dye-sensitized solar cells. In the following study, the traditional titanium dioxide particles used in DSCs will be replaced with nickel oxide nanowires to create a newer solar cell. It is hypothesized that the new augmented solar cell will result in an increase of the fill factor, open circuit voltage, and short circuit current, which ultimately improves its efficiency. METHODOLOGY NiO Synthesis Ni foils were taken and partitioned into 1x1 cm squares. These squares were then placed into LiOH solutions of different densities to soak overnight. The LiOH solutions were 15 mL and ranged from 5 g/L to 50 g/L in intervals of 5 g/L. After the foils were allowed to soak overnight, they were heated to 400ºC in air to allow NiO to form. To analyze the morphology of the NiO, scanning electron microscopy was used. This was repeated in several trials since the first trial did not yield optimal results. Only densities of 15 g/L, 30 g/L, and 50 g/L were used the second time since they yielded the most uniform results after the first trial. Also, some of the foils were pretreated mechanically or chemically to try to produce more uniform results. Three were treated with a 6.0M solution of HCl to burn off any impurities, another three were mechanically ground using a machine, and three served as controls. A final third trial was done with testing of NaOH, KOH, and LiOH at 30 g/L without pretreatment. Since none of the foils yielded nanowires, a different synthesis was tested. The goal for this synthesis was sea-urchin like particles of NiO. These particles were normal nanoparticles with spikes or wires coming out all around them. A solution was made from 2.333g NiCl2•6H2O in 14 mL of distilled water. Afterwards, 0.933g of urea was added to the solution. This solution was heated to 150ºC for 3 hours, and then cooled down to room temperature under running water. The blue precipitates were filtered off and washed with distilled water and absolute ethanol for several times, then dried in air at 100ºC for 8 hours. Finally, the obtained nickel precursors were calcined in air at 400ºC for 2 hours. Cell Construction A glass slide with FTO (fluorine-doped tin oxide) was taped onto a counter leaving only a .6cm x .6cm square unmasked in the center. Afterwards, 25 microliters of NiO paste was put onto the square. The NiO paste was synthesized by adding 1g of NiCl2 to 0.9g F-108 powder (commercially available surfactant). The powders were grinded and mixed together. 2.5g H2O and 5g of ethanol were added to the powder, and then it underwent sonification to dissolve out. It was put in the oven at 30ºC for 3 days, and then it was taken out and centrifuged. The paste was then doctor-bladed onto the substrate using the side of a clear glass slide to level it out. The slide was put into an oven and heated slowly to 450ºC in air for 30 minutes. Afterwards, a thin, rectangular strip of plastic adhesive was placed around the square of NiO in order to form a seal. A small hole was drilled into another glass slide with FTO and a thin layer of platinum. This slide was then placed on top of the other slide, and heated in order for the adhesive to melt and work. This created a seal between the seal and the NiO. By putting the cell into a vacuum, all excess air in the seal between the adhesive and NiO could escape, and an electrolyte of 1.0M LI and 0.1M I2 dissolved in 3-methoxypropionitrile filled the empty space left behind. Finally the hole was sealed airtight with the same adhesive and a thin glass slide. To make the augmented cell with the sea-urchin like particles, the same procedure was taken, except instead of adding NiCl2 to the NiO paste, the NiO powder was added. Every other part of the cell construction followed the same procedure. Efficiency Testing The solar cells were taken and attached with alligator clamps to a photovoltaic measuring device that shone ultraviolet light on the cell to test its performance. The cell’s voltage was compared to its current and the device gave a table of data and a graph for the performance of both cells. The cells were also tested in a dark setting to ensure that the energy produced was solely caused by the light. RESULTS The synthesis of the sea-urchin NiO particles was successful, as they had defined nanostructures and wires. Because of this successful synthesis, these particles were implemented onto a cell. The augmented cell with the NiO sea-urchin nanostructure film was compared to a control cell with a NiO spherical nanoparticle film. FIGURE 1 Figure 1 shows the sea-urchin like particles under SEM, or scanning electron microscopy. They are of different magnifications, but it can clearly be seen that there are nanowires protruding from the particles. After these particles were synthesized, they were utilized in a film that would ultimately be used to make a solar cell. These particles were directly grown onto the FTO slide through a hydrothermal reaction. The cell assembled with that film was compared to a different DSSC with a film of traditional spherical NiO nanowires. The voltage and the current were graphed and compared. The results show that the cell with the synthesized particles had a higher short circuit voltage and open circuit current. TABLE 1 Cells F108 NiO/O3 Dye Light DirectGrowth NiO/O3 Dye Light Film thickness (micron) Jsc (mA/cm^2) Voc (V) Fill Factor Efficiency (%) 1.3 1.33 0.111 0.387 0.057 1.7 1.73 0.114 0.364 0.072 Table 1 shows the major points of data for both cells. By using the short circuit current and the open circuit voltage, the fill factor was calculated and all of these were used to calculate the efficiency of the cells. The final result was that the augmented cell did perform better than the standard cell. FIGURE 2 Figure 2 is the graph given by the data collection device of how both cells performed. It can be seen here that the augmented cell with the sea-urchin particles truly did perform better, and that both cells were successfully made since they were also tested in a dark setting, to make sure that it was truly the ultraviolet rays that were being converted into electricity. Although both cells functioned well, the cell with direct growth on the FTO with the sea-urchin like particles seemed to perform better. DISCUSSION AND CONCLUSION This study resulted in numerous different results. The hypothesis was proven to be correct because the NiO DSSC that utilized the enhanced sea-urchin nanowire particles performed better than the standard NiO nanoparticle DSSC. This was because it had a higher short circuit current and open circuit voltage, which was most likely due to improved separation of charge carriers, and improved transport of these charge carriers through the nanowire structures. Another success that this study had was the synthesis of the sea-urchin nanoparticles along with a successful creation of a cell with the particles. A new method of utilizing the particles was used by growing it directly onto the FTO substrate in a hydrothermal reaction chamber instead of first making a film with it. The process is not exact yet and is still unrefined, but through more work could be a promising path into better construction of DSSCs in the future. Future studies that could be done of this study include expanding into other areas of a DSSC. For example, instead of focusing on the semiconductor, the electrolyte or dye could be tested to see if any improvements could be made to overall efficiency based on those. Also, work could stay restricted with semiconductors, but next time return to the original idea of attempting to synthesize organized nanowires or work with different semiconductors. There are numerous ways to expand the study in terms of DSSCs, but even more possibilities are out there in the ever expanding field of solar energy. REFERENCES Grätzel, Michael. “Dye-sensitized Solar Cell Review.” Journal of Photochemistry and Photobiology 4 (2003): 145-53. PDF file. Law, Matt, et al. “Nanowire Dye-sensitized Solar Cells.” Nature Materials 4 (2005): 45559. Nature Materials. Web. 26 Oct. 2012. <http://www.nature.com/nmat/journal/v4/n6/abs/nmat1387.html>. Mintz, T. S., et al. “Electrochemical Synthesis of Functionalized Nickel Oxide Nanowires.” Electrochemical and Solid State Letter 8.9 (2005): 26-30. The Electrochemical Society. Web. 26 Oct. 2012. <http://esl.ecsdl.org/content/8/9/D26.short>. Pang, Huan, et al. “Selective Synthesis of Nickel Oxide Nanowires and Length Effect on Their Electrochemical Properties.” Nanoscale 2.6 (2010): 920-22. RSC Publishing. Web. 26 Oct. 2012. <http://pubs.rsc.org/en/content/articlelanding/2010/nr/c0nr00027b/unauth>. Xia, Younan, et al. “One-Dimensional Nanostructures: Synthesis, Characterization, and Applications.” Advanced Materials 15.5 (2003): 353-89. PDF file.