Want to make a battery
advertisement

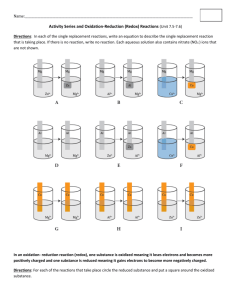
Want to make a battery? All you need are several lemons, other fruits, or fruit juices, two different kinds of metal, and some pieces of wire for connecting everything together. Once you have those items, you need only a little knowledge of electrochemistry and you’ll be ready to go. How does a fruit battery produce electrical energy? The fruit itself is a container for a solution of electrolyte. As you may know most fruit juice is acidic. The hydrogen ions provide the conduction of charge through a lemon battery. Two dissimilar metal strips are electrodes at which an oxidation reaction and a reduction reaction take place to provide the batteries power source. Two different chemical reactions take place at each of the metal strip electrodes. The electrode with the metal that is more easily oxidized becomes the anode, the electrode at which the oxidation reaction occurs. The second electrode becomes the cathode, and the reduction reaction proceeds at its surface. When these two reactions occur together, in the same cell, they combine to produce a spontaneous redox reaction. This spontaneous reaction generates the cell voltage of a battery by producing a different electrical potential and each electrode. Oxidation Potential (V) Easily Oxidized Li K Ca Na Mg Al Mn Zn Cr Fe Ni Sn Pb Cu Ag Hg Pt Au Not Easily Oxidized 3.04 2.92 2.87 2.71 2.37 1.66 1.18 0.76 0.74 0.44 0.25 0.14 0.13 -0.16 -0.78 -0.80 -1.20 -1.50 Using the information in the table to the left answer the following questions: 1. What are three metals that are easily oxidized? 2. What are three metals that are not easily oxidized? 3. How does the oxidation potential relate to the ease of oxidation of a metal? 4. Chemical cells depend on a difference of oxidation potential in the two electrodes. Knowing this, would a battery made with a nickel and a tin electrode be very effective? What would be a better choice? Now that you have an understanding of the way a battery works, lets try to use different fruits and electrodes to make some batteries! Instructions for the lab will be found at your lab station along with your supplies. Be sure to read the instructions carefully so that your data will be accurate. The data table below will be where you will record your data. Fruit (electrolyte) Lemon Lemon Lemon Lemon Lemon Lemon Lemon Lemon Lemon Lemon Lemon Lemon Orange Orange Orange Orange Orange Orange Orange Orange Orange Orange Orange Orange Tomato Tomato Tomato Tomato Tomato Tomato Tomato Tomato Tomato Tomato Tomato Tomato Anode (Red) Aluminum Aluminum Aluminum Zinc Zinc Zinc Nickel Nickel Nickel Copper Copper Copper Aluminum Aluminum Aluminum Zinc Zinc Zinc Nickel Nickel Nickel Copper Copper Copper Aluminum Aluminum Aluminum Zinc Zinc Zinc Nickel Nickel Nickel Copper Copper Copper Cathode (Black) Zinc Nickel Copper Aluminum Nickel Copper Aluminum Zinc Copper Aluminum Zinc Nickel Zinc Nickel Copper Aluminum Nickel Copper Aluminum Zinc Copper Aluminum Zinc Nickel Zinc Nickel Copper Aluminum Nickel Copper Aluminum Zinc Copper Aluminum Zinc Nickel Prediction Voltage Follow Up Questions: 1. Which batteries produced the most electricity? 2. Knowing that we cannot make or destroy energy, where does the electrical energy come from? 3. Based on what you learned in class today design a battery that would be more effective then those that we tried in class today.