organic ionic
advertisement
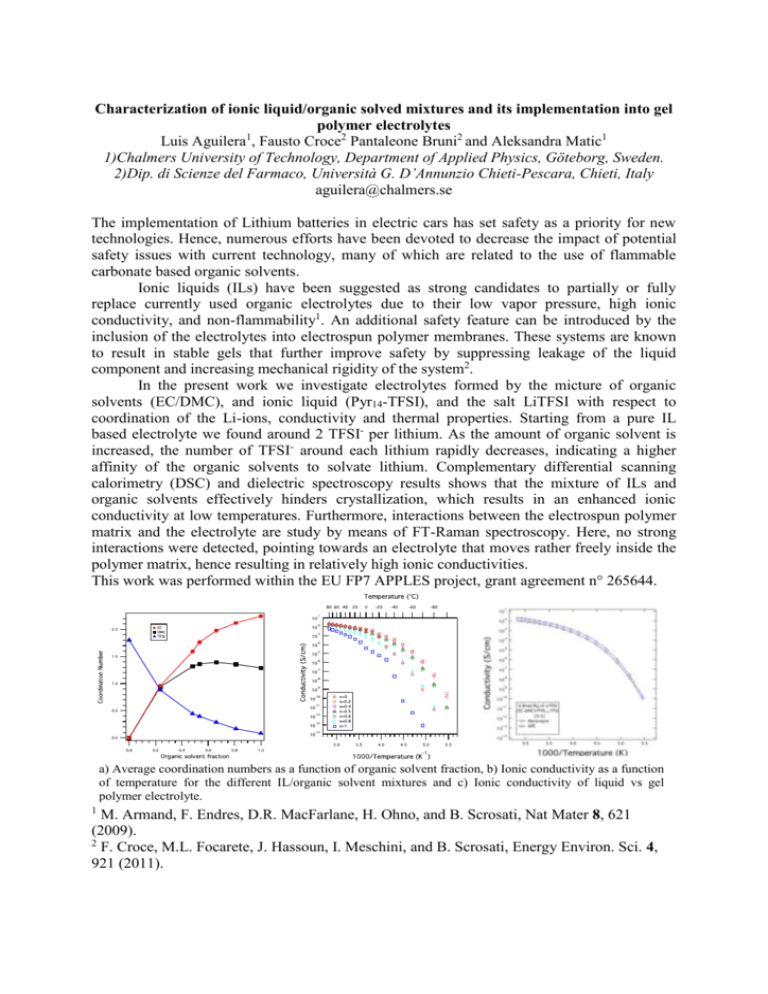
Characterization of ionic liquid/organic solved mixtures and its implementation into gel polymer electrolytes Luis Aguilera1, Fausto Croce2 Pantaleone Bruni2 and Aleksandra Matic1 1)Chalmers University of Technology, Department of Applied Physics, Göteborg, Sweden. 2)Dip. di Scienze del Farmaco, Università G. D’Annunzio Chieti-Pescara, Chieti, Italy aguilera@chalmers.se The implementation of Lithium batteries in electric cars has set safety as a priority for new technologies. Hence, numerous efforts have been devoted to decrease the impact of potential safety issues with current technology, many of which are related to the use of flammable carbonate based organic solvents. Ionic liquids (ILs) have been suggested as strong candidates to partially or fully replace currently used organic electrolytes due to their low vapor pressure, high ionic conductivity, and non-flammability1. An additional safety feature can be introduced by the inclusion of the electrolytes into electrospun polymer membranes. These systems are known to result in stable gels that further improve safety by suppressing leakage of the liquid component and increasing mechanical rigidity of the system2. In the present work we investigate electrolytes formed by the micture of organic solvents (EC/DMC), and ionic liquid (Pyr14-TFSI), and the salt LiTFSI with respect to coordination of the Li-ions, conductivity and thermal properties. Starting from a pure IL based electrolyte we found around 2 TFSI- per lithium. As the amount of organic solvent is increased, the number of TFSI- around each lithium rapidly decreases, indicating a higher affinity of the organic solvents to solvate lithium. Complementary differential scanning calorimetry (DSC) and dielectric spectroscopy results shows that the mixture of ILs and organic solvents effectively hinders crystallization, which results in an enhanced ionic conductivity at low temperatures. Furthermore, interactions between the electrospun polymer matrix and the electrolyte are study by means of FT-Raman spectroscopy. Here, no strong interactions were detected, pointing towards an electrolyte that moves rather freely inside the polymer matrix, hence resulting in relatively high ionic conductivities. This work was performed within the EU FP7 APPLES project, grant agreement n° 265644. a) Average coordination numbers as a function of organic solvent fraction, b) Ionic conductivity as a function of temperature for the different IL/organic solvent mixtures and c) Ionic conductivity of liquid vs gel polymer electrolyte. 1 M. Armand, F. Endres, D.R. MacFarlane, H. Ohno, and B. Scrosati, Nat Mater 8, 621 (2009). 2 F. Croce, M.L. Focarete, J. Hassoun, I. Meschini, and B. Scrosati, Energy Environ. Sci. 4, 921 (2011).











