Artigo Completo - Clique Aqui
advertisement

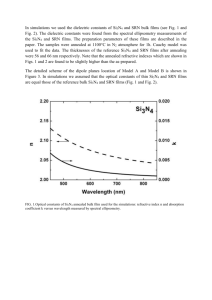
Anais do 43º Congresso Brasileiro de Cerâmica 2 a 5 de junho de 1999 - Florianópolis – S.C. 45901 SURFACE MODIFICATION AND AMMONIUM POLYACRYLATE ADSORPTION ON Si3N4 POWDERS María P. ALBANO*, Liliana B. GARRIDO* and Ana B. GARCIA**. *Centro de Tecnología de Recursos Minerales y Cerámica (CETMIC), C.C. 49 (1897) M. B. Gonnet, Provincia de Buenos Aires, ARGENTINA. Fax 54 21710075, e-mail:cetmic@netverk.com.ar. **Instituto Nacional del Carbon (CSIC), La Corredoria, s/n. Apartado 73. 33080 Oviedo, ESPAÑA. Fax 98 5297662, e-mail: anabgs@muniellos.incar.csic.es. ABSTRACT A precipitate coating of Al(OH)3 on Si3N4 particles was shown to improve the adsorption of ammonium polyacrylate (NH4PA) dispersant on Si3N4. The coated powders showed isoelectric points at pH 7.5 close to that of Al(OH) 3, indicating that the surface coverage was almost complete. The coating of Al(OH)3 on Si3N4 particles resulted in a bayerite-like surface with a small amount of gibbsite-like structure which improved the adsorption of a NH4PA dispersant. The maximum adsorption at pH 9.5 increased from 0.01 to 0.075 mg/m 2 for the as-received and coated Si3N4 powders, respectively. Key words: Si3N4, adsorbed Al(OH)3 , bayerite. 1 INTRODUCTION Sintered silicon nitride is an example of a multicomponent system since sintering agents (Y2O3 and Al2O3) are required to give a liquid phase during densification. The dispersion of Si3N4 particles is a critical step in the colloidal processing of the powder. Ceramic slips can be stabilized electrosterically by adsorption of polyelectrolyte additives such as ammonium polyacrylate (NH4PA) (1). However, a surface treatment of the Si3N4 powder is necessary to enhance the specific adsorption of the NH 4PA (2,3). Therefore, to help the dispersion of the silicon nitride powder with NH 4PA, a surface modification was attempted. 45902 Anais do 43º Congresso Brasileiro de Cerâmica 2 a 5 de junho de 1999 - Florianópolis – S.C. The approach that we took was to coat the silicon nitride with an hydroxide. Since sintering aids are needed, it would be desirable that the surface coating could also be a sintering aid as well, so that it not only improves the dispersion of silicon nitride but also has the potential to act as a sintering aid. Therefore, a precipitate coating of Al(OH) 3 was chosen. The coated Al(OH)3 layer can be transformed to alumina by heating, which is one of the sintering aids for silicon nitride. The purpose of this study then was to investigate the influence of the Al(OH)3 coating on the amount of NH4PA adsorbed for 11.8 vol% aqueous Si3N4 slips. The adsorption isotherms of the uncoated and coated powders at pH 9.5 were determined. 2 EXPERIMENTAL 2.1 Materials. A commercial -Si3N4 powder (SN-E10, UBE Industries, Japan) was used in this work. The mean particle diameter and the specific surface area were 0.6 m and 10 m2/g, respectively. The Al(OH)3 - precursor solution used to coat the Si3N4 particles was prepared by adding NaOH very slowly to an AlCl3 solution for a NaOH/Al mole ratio of 2.1. This partially neutralized clear solution contains a positively charged OH-Al polymer commonly referred to as the “Al13 polymer” (4). The Al concentration of the solution was 0.02 M. At a NaOH/Al mole ratio of 3 (pH 9), all the Al ions become completely neutralized, and the Al13 polymer rapidily converts to Al(OH)3 (4). 2.2 Coating method. The Si3N4 powder was dispersed in deionized water at about pH 10 to form a 9.4 vol% (25 wt%) slurry and ultrasonicated for 10 min to break down agglomerates. Then, 25 ml of the Al13 polymer solution was added while the slurry was stirred, and the pH adjusted at 9 by additions of NH3 (25 %) to precipitate Al(OH)3. This process was repeated several times to produce Si3N4 particles coated with various amounts of Al(OH)3. The pH 9 was maintained during the additions. The suspension of the coated particles was centrifuged. The solid was then recovered, washed with distilled water and dried at 100 C. The content of the Al(OH)3 coatings is expressed in this paper as the equivalent concentration of Al2O3. Si3N4 powders coated with an equivalent concentration of 2 and 3 wt% Al2O3 were prepared. Anais do 43º Congresso Brasileiro de Cerâmica 2 a 5 de junho de 1999 - Florianópolis – S.C. 45903 2.3 Powder characterization. The as-received and the coated Si3N4 powders were characterized by Fourier transform infrared (FTIR) analysis (Bruker IFS 66, Germany). 2.4 Zeta potential determinations. The electrophoretic mobilities of the as-received and the coated Si3N4 powders was measured in a Penkem Laser Zee Meter 501, USA and converted into zeta potentials according to Smoluchowski's equation. For each determination, 0.05 g of sample were dispersed in 100 ml of 10 -2 M NaCl solution and the slurry stirred magnetically for 10 min before the measurements were carried out. To generate zeta potential versus pH curves and from them the IEP (isoelectric point), the pH of the slurry was adjusted using dilute HCl and NaOH solutions. 2.5 Slip preparation. Slips with a solid loading of 11.8 vol% (30 wt%) were prepared. A commercial ammonium polyacrylate solution (Dolapix CE 64, Zschimmer & Schwarz) with 36.5 % by weight of ammonium polyacrylate was used as the deflocculant. The pH of the suspension was adjusted with ammonia (25 %). Slips of coated and uncoated Si3N4 powders with various amounts of deflocculant were prepared by suspending particles in deionized water via 20 min of ultrasound. The pH was adjusted at 9.5. 2.6 Ammonium polyacrylate adsorption measurements. In order to determine the amount of ammonium polyacrylate adsorbed, slips were centrifuged for 30 min at 2500 rpm and washed once with distilled water. Afterwards, the solid was dried at 100 C and analyzed by thermal gravimetric analysis (TGA) (Model STA 409, Netzsch Inc., Germany) at a heating rate of 10 C/min in a N2 atmosphere. The TGA data (Fig. 1) showed a four-step pyrolysis. The first weight loss (20-120 C) was attributed to the removal of free water, the second (120-220 C) to the removal of chemically bound water, the third (220 –350 C) to the transformation of Al(OH)3 to Al2O3, and the final weight loss (350-800 C) due to the decomposition of ammonium polyacrylate. This weight loss was used to determine the amount of NH 4PA on adsorbed on each sample. Anais do 43º Congresso Brasileiro de Cerâmica 2 a 5 de junho de 1999 - Florianópolis – S.C. 45904 Figure1: DTA,TG and DTG curves of the coated Si3N4 powder with NH4PA adsorbed 3 RESULTS AND DISCUSSION 3.1 Powder characterization. The presence of Al(OH)3 on the Si3N4 particle surface was supported by FTIR analysis in Figure 2, which shows the spectra of the as-received and coated Si3N4 powders in the range of 3300-4000 cm-1. The as-received Si3N4 powder (a) did not show any band in this region, however a set of bands appeared in the spectra of the coated powders (b and c). Figure 2: FTIR spectra of different Si3N4 powders: (a) as-received, (b) 2wt% Al2O3 coated, (c) 3 wt% Al2O3 coated. 45905 Anais do 43º Congresso Brasileiro de Cerâmica 2 a 5 de junho de 1999 - Florianópolis – S.C. Each of the polymorphs of Al(OH)3 (gibbsite, bayerite and nordstrandite) have a series of characteristic OH-stretching bands in the IR region (4). The OH-stretching frequencies reported for well-crystalized gibbsite are at 3623, 3527, 3460, 3396 and 3384 cm-1 (4). Bayerite shows four OH-stretching bands at 3653, 3550, 3463 and 3435 cm-1 (4). Consequently, the observation of bands at 3652, 3621, 3548, 3527, 3464, 3440 and 3390 cm-1 for the coated Si3N4 powders (Fig. 2) could be considered as evidence for the presence of bayerite and well-cristallized gibbsite. Morever, the relative intensity of the bands indicated that the coated Si3N4 powders had a bayerite-like surface with a small amount of gibbsite-like structure. The intensity of the bands increased with an increase in the Al(OH)3 content of the coated powders “ as expected ”. 3.2 Zeta potential versus pH curves . Figure 3 shows the zeta potential versus pH curves of the as-received and coated Si3N4 powders. An IEP at pH 5.2 was found for the as-received Si3N4 powder. This IEP value was in agreement with that previously reported for this Si3N4 powder (5). As shown in Figure 3, the coating on the Si3N4 powder shifted the IEP value to higher pHs. The 2 wt% Al2O3 coated powder (b curve) showed an IEP at pH 7.5. An additional increase in the amount of coating up to 3 wt% Al 2O3 (c curve) had not effect on the IEP value. Figure 3: Zeta potential versus pH curves of different Si3N4 powders: (a) as-received, (b) 2 wt% Al2O3 coated, (c) 3 wt% Al2O3 coated. 45906 Anais do 43º Congresso Brasileiro de Cerâmica 2 a 5 de junho de 1999 - Florianópolis – S.C. The IEP of Al(OH)3 was found to be around 8 (5) similar to that of Al2O3 . Thus, the coating on the Si3N4 powder with 2 wt% Al2O3 shifted its IEP near that of Al(OH)3, indicating that at this concentration the surface coverage was almost complete. The negative zeta potential values of the coated powders increased monotonically with the suspension pH without reaching a plateau, evidencing that the particle surface charge was formed by the adsorption of ions from the solution. This effect was also noticeable in the positive zeta potential, the difference being that in this case the zeta potential value decreased with increasing pH. The presence of (-Al-OH) groups as terminal sites on the surface of Al(OH)3 can explain this behaviour (6). These type of groups are amphoteric in character, therefore they can adsorb either H or OH- from the solution to produce dominant positive (-Al-(OH)2) or negative (-Al-O -) surface sites below and above the IEP of the Al(OH)3, respectively. At the pH studied (9.5) the zeta potential values of the uncoated and 2-3 wt% Al2O3 coated powders were –43, -40 and –38.5 mV, respectively. 3.3 Amount of NH4PA adsorbed. Figure 4 shows the amount of NH4PA adsorbed as a function of the amount of NH4PA solution added (dry weight basis of powder) at pH ( m g /m 2 ) 9.5 for the uncoated and coated powders. The diagonal line represented the adsorption A d s o rb e d behaviour that would occur if 100 % of the NH4PA added was adsorbed. 0.3 0.25 a b c N H 4 P A 0.2 0.15 0.1 0.05 0 0 0.2 0.4 0.6 NH4PA Solution Added (wt%) 0.8 1 Figure 4: Amount of ammonium polyacrylate (NH4PA) adsorbed as a function of the amount of NH4PA solution added at pH 9.5 for different Si3N4 powders: (a) as-received , 45907 Anais do 43º Congresso Brasileiro de Cerâmica 2 a 5 de junho de 1999 - Florianópolis – S.C. (b) 2 wt% Al2O3 coated, (c) 3 wt% Al2O3 coated. The diagonal line represents 100% of ammonium polyacrylate adsorption We have previously characterized the as-received Si3N4 powder surface by FTIR analysis in the diffuse reflectance mode (3). These results indicated that the Si3N4 surface consisted mainly of amine (=N-H) groups with very few silanol (Si-OH) functional groups. The ammonium polyacrylate dissociation according to the reaction CH3 (CH2 C CH3 )n (CH2 C COO- )n + NH4+ COO- NH4+ begins at pH 3.5; at pH values 8.5 the polymer charge is negative with the fraction dissociated () approaching 1 (1). The RCOO – groups of the deflocculant are adsorbed at the silanol sites of the Si3N4 powder surface (3). As the Si3N4 powder has very few silanol reactive groups towards the deflocculant, a very low adsorption of NH 4PA on the as-received Si3N4 powder was found (Fig. 4). In spite of the high negative zeta potential values of the coated powders (section 3.2) the coating of Al(OH)3 resulted in significantly greater adsorption of NH4PA dispersant (Fig. 4). Thus, the maximum adsorption at pH 9.5 increased from 0.01 mg/m2 for the uncoated powder to 0.075 mg/m2 for the coated powders. The greater adsorption of NH4PA on the coated powders could be attributed to the high affinity of the polyelectrolyte to the Al2O3-like surface. An acid-base reaction can occur between hydroxyl groups on alumina and –COOH groups on the NH4PA chains (7). The adsorption curves of the 2 and 3 wt% Al2O3 coated powders were similar. The amount of NH4PA adsorbed increased with increasing amounts of NH4PA solution added up to reaching an adsorption plateau at 0.46 wt% of NH4PA solution added. This plateau corresponded to the adsorption saturation limit of the polyelectrolyte on the coated powders at pH 9.5. The curves of the coated Si3N4 powders did not follow the 100 % adsorption line, thus the adsorption plateau was reached with an appreciable amount of NH 4PA remaining in solution. A barrier for adsorption increasingly was developed with increasing the amount of NH4PA solution added due to the electrostatic repulsion between the negative surface of the NH4PA-adsorbed coated Si3N4 particles and the Anais do 43º Congresso Brasileiro de Cerâmica 2 a 5 de junho de 1999 - Florianópolis – S.C. 45908 anionic polymer remaining in solution. This adsorption behaviour is not of the “highaffinity type” (1). The results showed that the adsorption values of the 2 and 3 wt% Al 2O3 coated powders were similar. Since pressureless sintering of silicon nitride requires the addition of about 3-4 wt% Al2O3 , the coated powder with an equivalent concentration of 3 wt% Al2O3 can be used for the processing of concentrated aqueous slips with NH4PA. 4 CONCLUSIONS Si3N4 powders coated with various aluminium hydroxides, expressed as alumina percentages, were prepared and dispersed in water with NH4PA (PA= polyacrylate) to produce 11.8 vol% slips. The coating of Al(OH)3 on Si3N4 particles resulted in significantly greater adsorption of NH4PA dispersant. The maximum adsorption of the polyelectrolyte on the as-received and 2-3 wt% Al2O3 coated powders at pH 9.5 was 0.01 and 0.075 mg/m2, respectively. The adsorption behaviour of the coated powders at pH 9.5 was not of the “high-affinity type” since the adsorption plateau was reached with an appreciable amount of NH4PA remaining in solution. REFERENCES (1) J. Cesarano III, I. A. Aksay, ”Stability of aqueous -Al2O3 suspensions with poly(methacrylic acid) polyelectrolyte”, J. Am. Ceram. Soc. 71, 4 (1988) 250. (2) M. J. Hoffman, A. Naget, P. Greil, G. Petgow, “Slip casting of SiC-whisker-reinforced Si3N4 “, J. Am. Ceram. Soc. 72, 5 (1989) 765. (3) M. P. Albano, L. B. Garrido, “Processing of concentrated aqueous silicon nitride slips by slip casting”, J. Am. Ceram. Soc. 81, 4 (1998) 837. (4) P.H. Hsu, in Minerals in Soil Environments (2nd Edition), ed. Soil Science Society of America (Madison, WI) (1989) ,p. 331. (5) L. Bergstrom, R. J. Pugh, “Interfacial characterization of silicon nitride powders“, J. Am. Ceram. Soc. 72, 1(1989) 103. (6) E. P. Luther, F. F. Lange, D. S. Pearson,”Alumina surface modification of silicon nitride for colloidal processing”, J. Am. Ceram. Soc. 78, 8 (1995) 2009. Anais do 43º Congresso Brasileiro de Cerâmica 2 a 5 de junho de 1999 - Florianópolis – S.C. 45909 (7) C. M. Incorvati, D. H. Lee, J. S. Reed, R. H. Condrate Sr.,“Obtaining dispersible bayer-process aluminas in water”, Am Ceram. Soc. Bull. 76, 9 (1997) 65.